 |
 |
- Search
| Ann Child Neurol > Volume 32(2); 2024 > Article |
|
Abstract
Purpose
Methods
Results
Conflicts of interest
Sang Ook Nam is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Notes
Author contribution
Conceptualization: YMK.
Data curation: YHJ.
Formal analysis: SHC.
Funding acquisition: HWY.
Methodology: HYJ and SJP.
Project administration: KHP and YMK.
Writing - original draft: YJL (Yoo Jung Lee) and YMK.
Writing - review & editing: JHK, YJL (Yun Jin Lee), SON, and YMK.
Fig. 1.
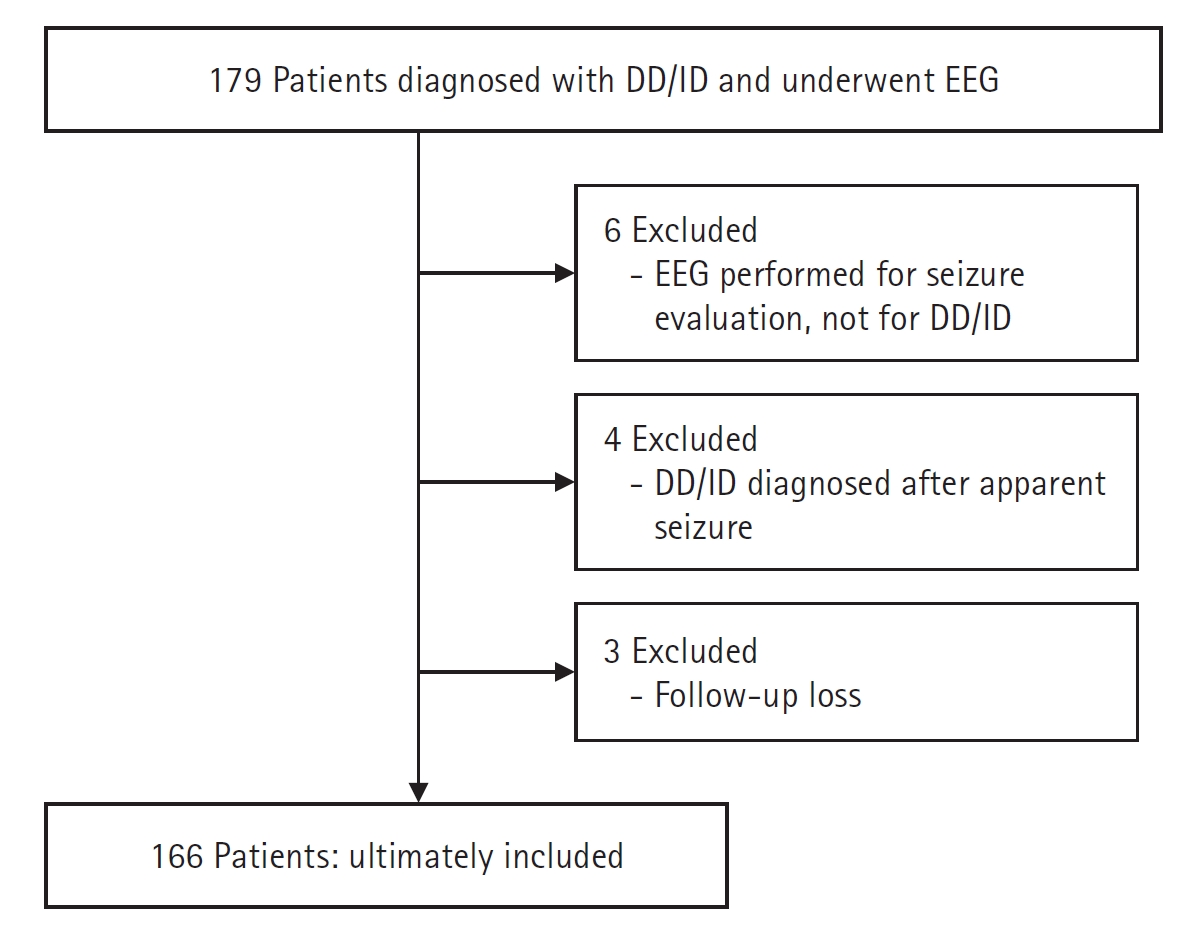
Table 1.
| Variable | Total (n=166) | Normal EEG (n=127) | Abnormal EEG (n=39) | P value |
|---|---|---|---|---|
| Sex (male/female) | 80/86 (48.2/51.8) | 65/62 (51.2/48.8) | 15/24 (38.5/61.5) | 0.164 |
| Age (yr) | 2.80±3.16 | 2.90±3.20 | 2.47±3.07 | 0.452 |
| 0-1 (infant) | 56 (33.7) | 36 (28.3) | 20 (51.3) | |
| 1-2 (toddler) | 27 (16.3) | 25 (19.7) | 2 (5.1) | |
| 2-5 (preschooler) | 58 (34.9) | 47 (37) | 11 (28.2) | |
| 5-10 | 18 (10.8) | 13 (10.2) | 5 (12.8) | |
| 10-15 | 5 (3) | 4 (3.1) | 1 (2.6) | |
| ≥15 | 2 (1.2) | 2 (1.6) | 0 | |
| Birth history | ||||
| Full term | 139 (83.1) | 108 (85) | 30 (76.9) | 0.236 |
| Preterm | 28 (16.9) | 19 (15) | 9 (23.1) | 0.236 |
| Perinatal complication | 53 (31.9) | 35 (27.6) | 18 (46.2) | 0.029a |
| ASD | 22 (13.3) | 17 (13.4) | 5 (12.8) | 0.927 |
| ADHD | 13 (7.8) | 8 (6.3) | 5 (12.8) | 0.185 |
| Epilepsy | 17 (10.2) | 9 (7.1) | 8 (20.5) | 0.016a |
| Laboratory results | ||||
| Lactic acid (mmol/L) | 1.91±1.06 | 1.85±1.0 | 2.09±1.24 | 0.354 |
| Pyruvic acid (mg/dL) | 0.87±0.51 | 0.88±0.56 | 0.84±0.33 | 0.579 |
| Ammonia (μg/dL) | 61.36±16.86 | 59.51±13.99 | 67.82±23.54 | 0.025a |
| CK (U/L) | 204.03±269.08 | 186.76±186.70 | 261.71±454.29 | 0.024a |
| TSH (μIU/mL) | 4.15±4.58 | 3.80±4.62 | 5.28±4.34 | 0.387 |
| fT4 (ng/dL) | 1.29±0.27 | 1.28±0.25 | 1.32±0.33 | 0.258 |
| Brain MRI abnormality | 61 (36.7) | 41 (32.3) | 20 (51.3) | 0.048a |
| Abnormal neonatal screening test via tandem mass spectrometry | 7 (4.2) | 5 (3.9) | 2 (5.1) | 0.214 |
| Single gene disorder | 11 (6.6) | 6 (4.7) | 5 (12.8) | 0.665 |
| Abnormal chromosome microarray (CNV-identified) | 77 (46.4) | 54 (42.5) | 23 (59) | 0.104 |
Values are presented as number (%) or mean±standard deviation.
EEG, electroencephalography; ASD, autistic spectrum disorder; ADHD, attention deficit hyperactivity disorder; CK, creatine kinase; TSH, thyroid-stimulating hormone; fT4, free thyroxine; MRI, magnetic resonance imaging; CNV, copy number variant.
Table 2.
Table 3.
| Characteristic | Genetically normal (n=83) | Genetically abnormal (n=83) | P value |
|---|---|---|---|
| Sex (male/female) | 41/42 | 39/44 | 0.756 |
| Abnormal EEG | 13 (15.7) | 26 (31.3) | 0.017a |
| Epilepsy | 9 (10.8) | 8 (9.6) | 0.798 |
| ASD | 14 (16.9) | 8 (9.6) | 0.170 |
| ADHD | 6 (7.2) | 7 (8.4) | 0.773 |
| Preterm infant history | 15 (18.1) | 13 (15.7) | 0.678 |
| Perinatal complication | 26 (31.3) | 27 (32.5) | 0.868 |
| Brain MRI abnormality | 30 (36.1) | 31 (37.3) | 0.217 |
Table 4.
Values are presented as number (%).
EEG, electroencephalography; FMR1, fragile X messenger ribonucleoprotein 1; PWS, Prader-Willi syndrome; AS, Angelman syndrome; PCR, polymerase chain reaction; MECP2, methyl-CpG binding protein 2; SMN1/2, survival motor neuron 1/2; NSD1, nuclear receptor binding SET domain protein 1; DMPK, dystrophia myotonica protein kinase; CNV, copy number variant; VUS, variant of unknown significance.
Table 5.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,149 View
- 29 Download
- Related articles in Ann Child Neurol
-
Significance of Polyspikes on Electroencephalography in Children with Focal Epilepsy2022 April;30(2)
Ischemic Encephalothy Found Accidentally in Children Diagnosed as Febrile Seizures.2006 May;14(1)
The Ictal Electroencephalographic Findings in a Neonate with Hypocalcemia Seizure.2007 May;15(1)
Clinical Aspects of Children with Developmental Stuttering.2012 June;20(2)






