Effect of Tizanidine on Spasticity in Pediatric Patients with Cerebral Palsy: A Systematic Review
Article information
Abstract
Purpose
Cerebral palsy (CP) is a permanent, non-progressive disorder of the developing brain. Tizanidine is an effective treatment for spastic CP; however, insufficient evidence exists regarding its effect on motor function and side-effect profile. This review explored the effects and safety of tizanidine in treating spasticity among pediatric patients with CP.
Methods
Two reviewers conducted a literature search. The Cochrane risk of bias tool and the 14-item National Institutes of Health Quality Assessment Tool were used to evaluate the risk of bias. A systematic review was performed for relevant studies.
Results
Five studies were included: three randomized controlled trials (RCTs) and two observational studies. The control group received a placebo in two RCTs, while baclofen was used in the remaining studies. Tizanidine dosage and duration varied across reports, except for the two observational studies. Excepting one observational study, tizanidine was associated with a greater improvement on the modified Ashworth scale. Pain reduction was also greater with tizanidine treatment compared to the placebo, as evidenced by one RCT. Three studies evaluating gross motor function reported superior results with tizanidine compared to baclofen. Two RCTs indicated similar safety profiles between tizanidine and the placebo. The remaining studies reported a more favorable safety profile for tizanidine than baclofen.
Conclusion
The studies examined in this review reported beneficial effects of tizanidine on spasticity, pain, and gross motor function. Tizanidine usage was associated with no serious adverse events, reflecting a better safety profile than baclofen. Nevertheless, high-quality RCTs are recommended to support tizanidine administration in pediatric patients.
Introduction
1. Description of the condition
Cerebral palsy (CP) is a group of disorders that affect the development of movement and posture, limiting activity. These disorders are linked to non-progressive disturbances in the developing fetal or infant brain [1]. Frequently, these disturbances also affect cognition, sensation, communication, and behavior. These conditions can potentially result in seizure disorders and secondary musculoskeletal complications such as contractures [1,2]. CP is categorized based on resting tone and the limbs affected. Spastic CP is the most common type, accounting for approximately 80% of cases [3]. Extrapyramidal or dyskinetic CP accounts for 10% to 15%, while ataxic CP makes up less than 5% of cases.
CP is acknowledged as the leading cause of childhood disability, impacting both function and development. Global population-based studies estimate its prevalence at 1.5 to over 4 cases per 1,000 live births [4-6]. In developing nations such as the Philippines, the prevalence of CP is not definitively known, but it is estimated to range from 1.5 to 5.6 cases per 1,000 live births [7].
The morbidity and mortality associated with CP are related to the severity of the condition and concomitant medical complications, including epilepsy, intellectual disability, and respiratory and gastrointestinal difficulties [8]. Approximately 25% of children with CP experience mild involvement, resulting in little to no functional limitation in ambulation, self-care, and other activities. About half are moderately impaired to the extent that complete independence is unlikely, but functionality remains satisfactory. Only around 25% of children are so severely disabled that they require extensive care and are non-ambulatory [9].
CP is managed through a multidisciplinary approach that encompasses physical, occupational, and nutritional therapy. This comprehensive approach is designed to enhance the academic and social integration of patients. However, in cases of spastic CP, spasticity may prove resistant, even with orthopedic and rehabilitative intervention. This often necessitates adjuvant pharmacologic treatment. The pharmacologic management of spasticity associated with CP includes treatments such as botulinum toxin injections, benzodiazepines, oral baclofen, tizanidine, dantrolene, and gabapentin [10,11]. Baclofen and tizanidine are particularly promising treatments for spasticity in CP due to their ease of administration and cost-effectiveness. While insufficient data currently exist to definitively support or refute the use of oral baclofen, it is widely used in a clinical context to treat spasticity in patients with CP.
2. Description of the intervention
Tizanidine, a centrally-acting alpha-adrenergic agonist, resembles diazepam and baclofen in its capacity to reduce muscle tone [12]. Its anti-nociceptive properties may further contribute to this tone-reducing effect, as pain is known to increase spasticity. While tizanidine may be effective [11], insufficient data exist regarding its impact on motor function improvement and its side-effect profile. A systematic review of tizanidine would be beneficial in consolidating available data on its effects and safety for pediatric patients with CP. Moreover, a systematic review can mitigate small sample sizes, a well-known limitation of many individual clinical studies.
3. Importance of this review
Any additional interventions to manage spasticity in pediatric patients with CP may enhance their quality of life. The present study could also benefit the caregivers and/or parents of these patients by addressing issues related to spasticity, such as difficulties changing diapers and moving the patient. Furthermore, clinicians who are constantly seeking methods to better assist their patients could gain valuable insights from a comprehensive review of the existing literature on this topic.
4. Objective
This review was conducted to evaluate the efficacy and safety of tizanidine in treating spasticity in pediatric patients with CP.
Materials and Methods
1. Research design
The authors carried out a systematic review to qualitatively synthesize relevant studies, in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. This study was undertaken from August 29 to September 29, 2022.
2. Study selection criteria for this review
1) Types of studies
To evaluate the impact and safety of tizanidine in pediatric patients, the authors incorporated all relevant observational and experimental studies. Articles that were excluded from this evaluation were (1) post hoc or secondary analyses of clinical trials; (2) case reports and case series; (3) editorials; (4) systematic reviews and/or meta-analyses; (5) review articles; and (6) abstracts for which the full-text articles were not accessible.
2) Types of participants
The participants in this study were pediatric patients, ranging in age from 6 months to 18 years, who had been diagnosed with CP and any degree of spasticity. No exclusions were implemented based on sex or ethnicity.
3) Types of interventions
For this review, the use of tizanidine was considered the intervention. No restrictions were in place regarding the dosage administered or the duration of treatment. Studies lacking a control or comparison group were excluded from the review.
3. Search methods for study identification
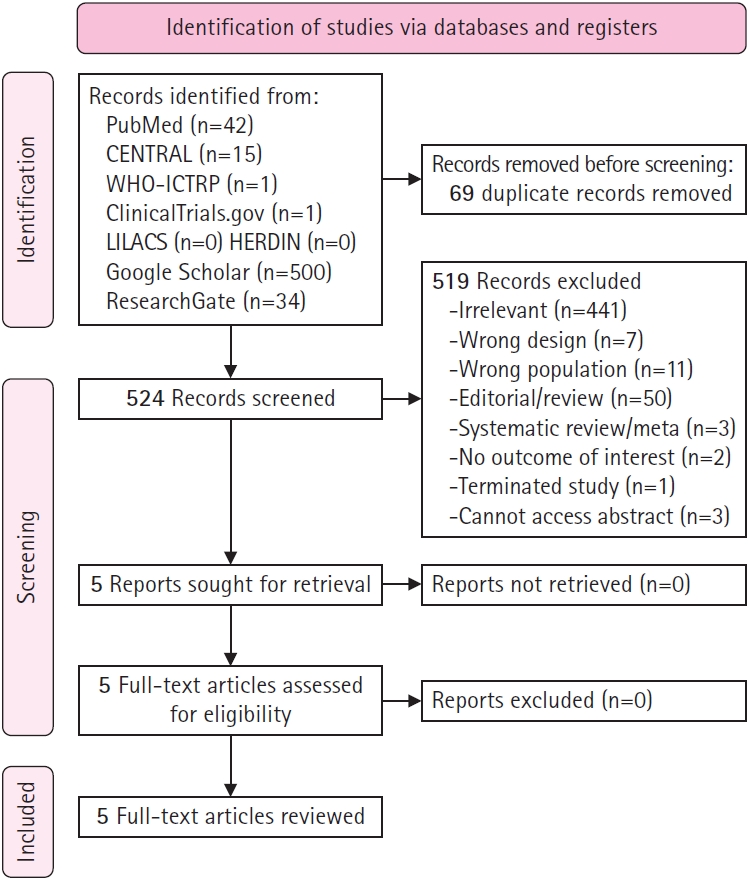
Two independent researchers, ADPC and MAAMV, performed a literature search for relevant studies using various electronic databases. These included PubMed, the Cochrane Central Register of Controlled Trials, the World Health Organization International Clinical Trials Registry Platform, Clinicaltrials.gov, the Latin American and Caribbean Health Sciences Literature, the Health Research and Development Information Network, Google Scholar (first 500 articles), and ResearchGate (first 100 articles) (Fig. 1). Additionally, the references of each study, including prior systematic reviews and meta-analyses, were examined to discover additional articles. The search utilized specific keywords and Medical Subject Headings terms, and Boolean operators were employed to broaden the search. These terms included “tizanidine,” “Zanaflex,” “sirdalud,” “child,” “pedia,” “cerebral palsy,” “spasticity,” “spastic,” and “spasm.” No restrictions were applied with regard to the publication date or language. Studies published up to August 29, 2022 were included.
4. Study selection
Duplicate studies were identified and excluded from the review using the Mendeley desktop application (Elsevier, Amsterdam, The Netherlands). Initially, two independent researchers (ADPC and MAAMV) screened the selected titles and abstracts for eligibility. Subsequently, these two authors independently evaluated the full-text articles of the chosen studies. Any discrepancies were addressed through discussion until a final decision was reached.
5. Risk of bias assessment of the included studies
One researcher (MAAMV), an experienced epidemiologist, utilized the Cochrane risk of bias tool integrated into Review Manager software (RevMan version 5.4, The Cochrane Collaboration, London, UK), to independently evaluate the risk of bias for each eligible randomized controlled trial (RCT). The assessment included the following areas, each of which was classified as having a low, unclear, or high risk of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Furthermore, the overall bias of each study was categorized as low if all key areas were deemed low risk, high if at least one key area was deemed high risk, and unclear if the study failed to meet any of the specified criteria.
For observational studies, the 14-item National Institutes of Health Quality Assessment Tool, specifically designed for observational cohort and cross-sectional studies, was used to evaluate the risk of bias in each included study. This tool is designed to assess the internal validity of each study by examining the potential risks of selection bias, confounding bias, measurement bias, and information bias. The quality ratings were defined as poor (<50%), fair (50% to 80%), and good (>80%).
6. Data collection process
Data extraction was performed by two independent reviewers (ADPC and MAAMV). The following information was obtained from each study and encoded in a standardized Excel (Microsoft Corp., Redmond, WA, USA) file: authors, year of publication, country, study design, sample size, and study outcomes. If available, demographic and clinical characteristics were also noted, such as age, sex, severity of spasticity, dosage of tizanidine, and duration of treatment.
7. Outcome variables and definitions
1) Primary outcome
The primary outcome was the proportion of participants who experienced a reduction in spasticity, as measured by the modified Ashworth scale (MAS). This was defined as a decrease of at least 1 point from the baseline score.
2) Secondary outcomes
• Mean MAS score
• Pain reduction after treatment
• Gross motor function after treatment
• Adverse events
8. Data analysis
A systematic review was conducted to qualitatively synthesize the data. The results were organized into tables. Due to the limited number of studies available, a meta-analysis was not performed.
9. Ethical considerations
The study utilized data from the findings of previously published research. Therefore, neither ethical approval nor patient consent was required.
Results
A total of 524 unique studies were reviewed based on their titles and abstracts. Three studies were excluded due to the unavailability of either an abstract or the full-text. From the reviewed abstracts, five full-text articles were selected and further scrutinized based on the eligibility criteria. All five articles met the criteria and were included in this systematic review. Fig. 1 (below) illustrates the process of study selection, along with the reasons for exclusion.
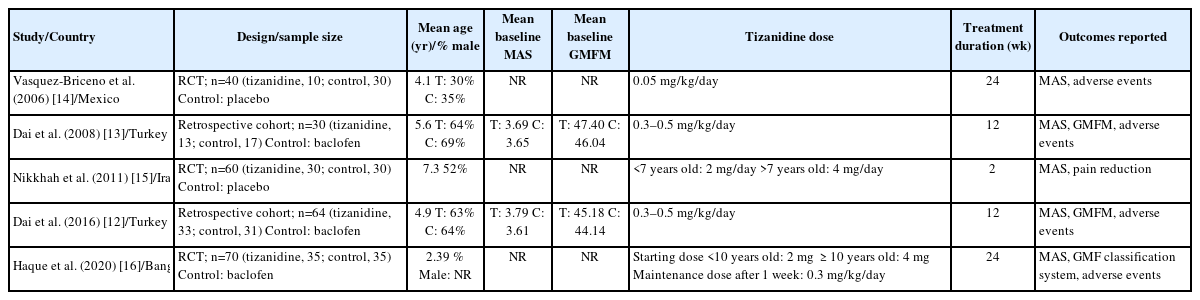
Table 1 outlines the characteristics of the studies included in this review, all of which were published between 2006 and 2020. These studies were conducted in various countries, namely Turkey (n=2), Mexico (n=1), Iran (n=1), and Bangladesh (n=1). Among the five studies, two were retrospective cohorts [12,13], while the rest were RCTs [14-16]. The sample size for each study varied from 30 to 70 participants, for a total sample size of 264. The mean age of participants ranged from 2.39 to 7.3 years. Male participants made up between 30% and 64% of the treatment group and between 35% and 69% of the control group. The mean baseline MAS and Gross Motor Function Measurement (GMFM) score were only reported in the studies conducted by Dai et al. [12,13] in 2008 and 2015. None of the studies provided information on the severity of spasticity. The dosage of tizanidine differed across the studies, with the exception of the two reports by Dai et al. [12,13] in 2008 and 2015. Two studies used a placebo for the control group [14,15], while the rest used baclofen [12,13,16]. The duration of treatment ranged from 2 to 24 weeks.
Table 2 provides a summary of the five studies reviewed.
1. Reduction in spasticity
In this review, the MAS was employed to evaluate the effects of tizanidine in alleviating spasticity in children with CP. Two RCTs were conducted, both utilizing a placebo as the control group [14,15]. Nikkhah et al. [15] noted a significantly larger percentage of patients exhibiting reduced spasticity in the treatment group (50%) compared to the control group (6.7%) (P<0.0001) following a 2-week follow-up period. Vasquez-Briceno et al. [14] found that the average improvement in MAS scores at the 24-week mark was greater in the treatment group than in the control group (78.85% vs. 7.644%, P=0.0001). Furthermore, score improvements associated with tizanidine were superior for shoulder flexors, elbow flexors, hand flexors, hip abduction, hip flexors, knee flexors, and foot dorsiflexion [14]. A detailed breakdown of improvements by site can be found in Table 2.
In the remaining RCT, baclofen was utilized as a control group, and the findings similarly indicated that tizanidine outperformed this control in terms of MAS improvement. In this particular study conducted by Haque et al. [16], the rate of MAS score reduction was more rapid in the tizanidine group compared to the baclofen group. By the conclusion of month 6, 46% of patients who received tizanidine treatment had a MAS score of 1, in contrast to fewer than 20% in the baclofen group [16].
The two observational studies [12,13] incorporated baclofen as the control group, with all patients receiving rehabilitation and local injections of botulinum toxin A, regardless of group assignment. By week 12, the MAS was lower in the treatment group compared to the control group. One study did not find a statistically significant difference between the two groups [12]. In the other, however, Dai et al. [12,13] presented evidence that the MAS significantly improved after administering botulinum toxin A in the tizanidine group, but not in the baclofen group.
2. Pain reduction after treatment
Only one study made a comparison between the pain reduction following treatment with tizanidine and a placebo. In the RCT conducted by Nikkhah et al. [15], a larger percentage of patients in the tizanidine group experienced pain reduction in their spastic upper or lower limbs compared to the control group (66.7% vs. 13.3%, P<0.0001).
3. Gross motor function after treatment
Three studies reported gross motor function after treatment, all of which utilized baclofen as the control group [12,13,16]. In the RCT conducted by Haque et al. [16], the researchers reported the proportion of patients across the five levels of the Gross Motor Function Classification Scale from the beginning of the study to the 6-month mark. A higher level indicated a greater degree of mobility limitation. By the end of the 6-month period, the majority of patients in the tizanidine group were categorized as levels 2 and 3 (76%). In contrast, more than half of the patients in the baclofen group remained at level 4 after 6 months [16].
Meanwhile, the two observational studies [12,13] employed the GMFM, with higher scores denoting a greater improvement in gross motor function. Both the tizanidine group and the control group (baclofen) demonstrated a significant increase in GMFM score at the 12-week mark compared to their baseline scores. However, the score of the tizanidine group at week 12 was significantly higher than that of the control group [12,13].
4. Adverse events
Two of the RCTs revealed no side effects in either the tizanidine or placebo groups [14,15]. In contrast, the RCT conducted by Haque et al. [16] reported a higher incidence of adverse effects in the baclofen group compared to the tizanidine group. However, the study did not provide further details about the specific adverse effects.
Similarly, in their retrospective cohort study, Dai et al. [13] reported that tizanidine had a superior safety profile compared to baclofen, although they did not provide specific percentages. Instead, they simply noted that the incidence rates of anorexia and abdominal pain were higher among patients in the baclofen group than in the tizanidine group [13].
The 2015 study by Dai et al. [12] was the sole study that detailed the adverse events experienced by the patients involved. Both the tizanidine and baclofen groups had reported instances of constipation, anorexia, lethargy, and fatigue. However, all of these symptoms were mitigated by the administration of botulinum toxin A. Participants in the baclofen group also reported dizziness, seizures, and nasal congestion. Given the higher frequency of adverse events in the baclofen group, the authors concluded that tizanidine demonstrated a marginally superior safety profile compared to baclofen [12].
5. Bias assessment
Of the three RCTs, only the 2011 study by Nikkhah et al. [15] demonstrated a low risk of bias across all evaluated parameters. Two of the three studies [14,16] presented an unclear risk of selection bias, as they did not provide details on random sequence generation and allocation concealment. However, all studies exhibited a low risk of performance bias, as patients were not aware of their treatment allocation. They also showed low risks of attrition and reporting bias. In contrast, the study by Haque et al. [16] posed a high risk of detection bias, as the outcome assessors were not blinded. It also presented a high risk of other biases due to the non-comparability of baseline characteristics such as age, potentially leading to confounding bias. The two retrospective cohort studies [12,13], however, were assessed as being of fair quality.
Discussion
The aim of this study was to evaluate the efficacy and safety of tizanidine in treating spasticity among pediatric patients with CP. Tizanidine is a Food and Drug Association-approved medication for managing spasticity, but its application in pediatric patients with CP is currently limited due to a lack of sufficient clinical evidence [17]. A systematic review conducted in 2010 investigated the pharmacological treatments available for pediatric patients with CP [18]. The authors of that review concluded that the evidence supporting the use of tizanidine was insufficient, as only one RCT was available at the time. However, the American Academy of Neurology has recognized that tizanidine may be beneficial in treating spasticity in children, but that more robust evidence is required to endorse its use [18]. In 2013, another systematic review examined the pharmacological treatments available for children with CP. Based on this review, the evidence supporting the use of tizanidine remained weak, as the authors referenced only a 2010 study by Delgado et al. [19]. Additionally, a review published in 2020, which discussed various interventions for CP, concluded that tizanidine is likely effective in reducing spasticity in children [11]. However, no further details about this conclusion were provided.
In this systematic review, the authors compiled the findings of published RCTs and observational studies that specifically employed tizanidine to treat children with CP. Although it was introduced in the 1990s as an anti-spastic agent, studies of tizanidine in children remain rare. These authors found only three RCTs, of which just one was deemed high-quality, along with two observational studies [14-16]. One potential explanation for its limited use in the pediatric population is that tizanidine is only commercially available in tablet form. A liquid formulation of the drug, which would be more suitable for children, is currently under development and investigation [20,21].
Except for an observational study by Dai et al. [12] in 2015, all of the studies included in this review consistently demonstrated that tizanidine was more effective than the control—either placebo or baclofen—in reducing muscle tone as measured by the MAS score. Even with as little as 2 weeks of treatment, a significant improvement in spasticity has been noted [15]. However, the variations in the endpoint for measuring the MAS score prevented the execution of a meta-analysis in this review. Some studies reported the difference in the MAS score before and after treatment [12,13], while others reported the proportion of participants with a reduced score [14-16]. Nevertheless, all studies provided valid endpoints that could serve as a foundation for future research.
Tizanidine, an adrenergic agonist and muscle relaxant, inhibits the presynaptic release of norepinephrine and exerts an inhibitory effect on alpha motor neurons [17,22]. Additionally, its activity at the postsynaptic amino acid and imidazoline receptors leads to a reduction in the facilitation of spinal motor neurons [12]. These mechanisms may account for the positive effect of tizanidine in reducing spasticity in patients with CP.
Researchers have also hypothesized that the antinociceptive effect of tizanidine, which is mediated through the release of substance P in the spinal cord, contributes to its ability to reduce muscle tone [12]. Pain is positively correlated with spasticity in CP cases [23], which lends support to this hypothesis. This review also revealed that the tizanidine group experienced a greater reduction in pain than the placebo group. However, this has only been shown in one study involving a pediatric population [15].
Two studies included in this systematic review combined oral medications for CP with botulinum toxin A [12,13]. Combining treatments for CP is quite common, as this approach can increase the clinical effect while minimizing side effects [24]. Botulinum toxin A is a well-researched treatment for CP, even among pediatric patients [11,25]. However, the need for frequent injections makes it less convenient than oral medications, particularly for children. The two observational studies concluded that, despite both groups receiving botulinum toxin A, tizanidine was more effective in improving spasticity and gross motor function, and it had fewer adverse events compared to baclofen [12,13].
In this systematic review, three studies investigated the impact of tizanidine on gross motor function in addition to its effect on spasticity. While both baclofen and tizanidine were found to enhance gross motor function, all authors concluded that tizanidine was more effective than baclofen. The significant reduction in spasticity brought about by tizanidine could account for the observed improvement in gross motor function in children with CP. This conclusion is supported by previous studies that have established a correlation between these two measures [26,27].
A primary concern associated with tizanidine usage is its potential for adverse hepatic events, which is why liver function monitoring is often recommended [14]. However, in the present study, no serious adverse events were reported in patients who received tizanidine. In the RCT conducted by Vasquez-Briceno et al. [14], no changes in liver function tests were found at the conclusion of the study period. The 2015 study by Dai et al. [12] offered insights into the side-effect profiles of both baclofen and tizanidine. They found that lethargy, fatigue, constipation, and anorexia occurred more frequently in patients treated with baclofen than in those treated with tizanidine [12]. In a previous study by the same authors, the combination of botulinum toxin A and tizanidine resulted in greater effectiveness and fewer side effects than the combination of botulinum toxin A and baclofen [13]. The authors hypothesized that botulinum toxin A could reduce these adverse effects by allowing for a decreased dosage of oral anti-spasticity medications [12].
Despite the good safety profile of tizanidine, proper dosing is still warranted, especially in children. An overdose of this medication can be life-threatening. For instance, a case report documented a 2-year-old child with CP experiencing liver, kidney, and cardiovascular failure due to a tizanidine overdose. However, that report involved a dose of 1.6 mg/kg/day, which is higher than the dosage used in the studies included in this systematic review [28].
1. Limitations
Due to the limited evidence, tizanidine remains an off-label drug for pediatric patients with CP. The present systematic review underscores the fact that, despite being available for over two decades, only a handful of high-quality studies have been published examining the effects of tizanidine on children with CP. Furthermore, the quality of evidence provided by this study remains low, as only three RCTs have been conducted to date. Among these RCTs, only one demonstrated a low risk of bias.
The heterogeneity in dosage, follow-up, control groups, and endpoints in the available research hindered the execution of a meta-analysis. Despite the availability of two observational studies, the evidence produced in these investigations cannot be combined with that of RCTs.
2. Conclusion
Despite the positive outcomes observed for tizanidine, it is important to note that the evidence supporting these results remains low-quality, as indicated by the present systematic review. The data suggest that tizanidine outperforms both placebo and baclofen in reducing spasticity, relieving pain, and improving gross motor function in children with CP. Furthermore, tizanidine demonstrated a favorable safety profile with no reported serious adverse events, surpassing the safety of baclofen. However, it remains prudent to carry out high-quality RCTs to gather more solid evidence concerning the use of tizanidine in children with CP.
3. Recommendations
1. High-quality RCTs with standardized doses, treatment durations, and endpoints are still required to support the use of tizanidine in the pediatric population.
2. Long-term follow-up periods and examinations of other relevant endpoints, such as quality of life and gait, are recommended to further clarify the benefits of tizanidine use.
3. Future studies should also explore the use of the liquid formulation of tizanidine, specifically developed for children.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: ADPC and MAAMV. Formal analysis: ADPC. Methodology: ADPC and MAAMV. Writing-original draft: ADPC and MAAMV. Writing-review & editing: ADPC and MAAMV.