Clinical Differences between Enterovirus and Human Parechovirus in Children and Infants
Article information
Abstract
Purpose
Enteroviruses (EVs) and human parechoviruses (HPeVs) are important pathogens that cause fever in young infants and meningitis in children and have similar clinical symptoms and characteristics. The aim of this study was to compare the clinical symptoms and characteristics of EV and HPeV infections in young children and infants.
Methods
From June to August 2018, we obtained 50 cerebrospinal fluid (CSF) samples, of which 36 and 14 were EV- and HPeV-positive, respectively, as determined by film array methods. We then compared the clinical characteristics and laboratory values of patients with EV and HPeV infections.
Results
HPeV patients had a lower age than EV patients, but had similar sex predominance and fever duration. Moreover, EV patients had a higher prevalence of headache and mannitol use than HPeV patients. The blood and CSF white blood cell counts were lower among HPeV patients even after adjusting for sex and age. Furthermore, both viruses were found to cause occasional transient white matter injuries in the brain.
Conclusion
The clinical characteristics of HPeV and EV infections were found to be generally similar, but with a few noteworthy differences.
Introduction
Aseptic meningitis is commonly caused by enterovirus (EV) infection, which is mostly a benign pathogen with a few exceptions in all ages, especially in children [1-3]. Human parechovirus (HPeV) previously belonged to the enterovirus genus in the Picornaviridae family but was re-classified to the parechovirus genus [4]. HPeVs share many biological, clinical, and epidemiologic characteristics with EVs [5-7]. Both viruses are also common potential pathogens for neonatal febrile disease [8,9] and febrile disease with upper respiratory tract infection or viral exanthema [10]. Both viruses are transmitted via the fecal-to-oral route and all age groups are susceptible. Although both viruses have similar clinical symptoms, some studies have reported difficultly in differentiating them based on their clinical characteristics alone, especially in young infants without symptoms [3].
Both EV and HPeV infections have similar clinical findings and are the main leading causes of meningoencephalitis (ME) in neonates, and can sometimes cause fatal diseases, especially in infants and neonates. EV infections, especially those of the E71 virus, have been reported to be fatal in children, with few cases of endemic encephalitis and brain stem encephalitis. Recently, HPeV has also been reported to cause white matter disease in neonates with some cases being fatal [5,11-13] or having a poor prognosis in the long term follow-up.
This study aimed to compare the clinical aspects of EV and HPeV infections. Moreover, the findings of this study will help to better manage patients and their treatment and inform future studies.
Materials and Methods
1. Patient enrollment, study population, and study design
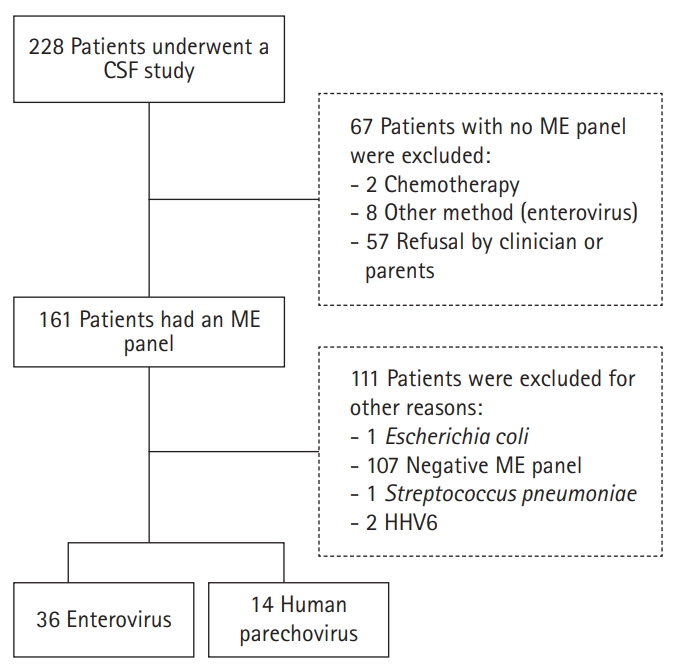
This retrospective chart review study was performed on children who underwent cerebrospinal fluid (CSF) examination in the CHA Bundang Hospital for ME diagnosis due to fever or neck stiffness symptoms from 1st June 2018 to 31st August [10,14-17]. We enrolled 228 patients all below 15 years of age who underwent lumbar puncture. The exclusion criteria were: (1) incomplete CSF studies such as traumatic tap; (2) non-meningitis causes of fever with a defined alternative fever focus; or (3) lumbar puncture for diagnosis of metastatic cancer or for delivering cancer medication (Fig. 1). After exclusion, 161 samples were analyzed for the detection of virus using Film Array® ME panel (bioMerieux SA, Marcy-l'Étoile, France). Among these samples, 50 were positive for either HPeV or EV, and we compared the clinical symptoms, laboratory findings, treatment, and prognosis of the corresponding patients.
2. Demographical characteristics and clinical/laboratory information
We retrospectively reviewed the medical records of the HPeV- and EV-positive patients and collected data such as age, sex, symptoms (e.g., duration of fever, presence of vomiting, headache, and irritability), and treatment (e.g., administration of hypertonic fluid [mannitol] and immunoglobulin). Furthermore, we collected their laboratory results such as hemoglobin, white blood cell (WBC) count and its differential count, platelet count, and erythrocyte sedimentation rate (ESR) as well as CSF examination findings such as CSF total and differential WBC count, pH, protein levels, and glucose. Subsequently, we adjusted the findings according to age and sex. Lastly, we collected and analyzed brain scan images where available. The CSF WBC was corrected according to the CSF red blood cell (RBC) count if the CSF RBC count was more than 50,000 cells/mm3. In cases where the CSF WBC count was more than 10 cells/mm3, we used the differential WBC count. In cases where the patient underwent multiple lab tests, such as for C-reactive protein (CRP) and WBC count, the highest values were used for statistical evaluation.
3. Virus detection in CSF study: ME panel
We assessed the CSF samples for the viruses using the Film Array® ME panel from bioMerieux, which requires 200 μL of CSF and takes about an hour to complete. We used freeze-dried reagents to detect the nucleic acids of the particular pathogens. This method allowed detection of all species of EV (A–D) and several serotypes of human EV including EV71, EV68, coxachieviruses, and echoviruses. Moreover, it allowed detection of HPeV serotypes 1–6 [18].
4. Statistical analysis
Data were analyzed using the chi-square test, Fisher’s exact t-test, Mann-Whitney test, and multiple logistic or linear regression using SPSS version 23.0 (IBM Co., Armonk, NY, USA). Multivariable regression models were used to estimate the adjusted odds ratios and 95% confidence intervals adjusted for age and sex. A P value of less than 0.05 was considered statistically significant. The Mann-Whitney test was used to analyze variables that did not show a standardized distribution after the Kolmogorov-Smirnov test.
5. Ethics statement
The study protocol was approved by the appropriate Institutional Review Board of CHA University (CHAMC-2018-08-011). Informed consent was waived by the board.
Results
1. Demographic findings
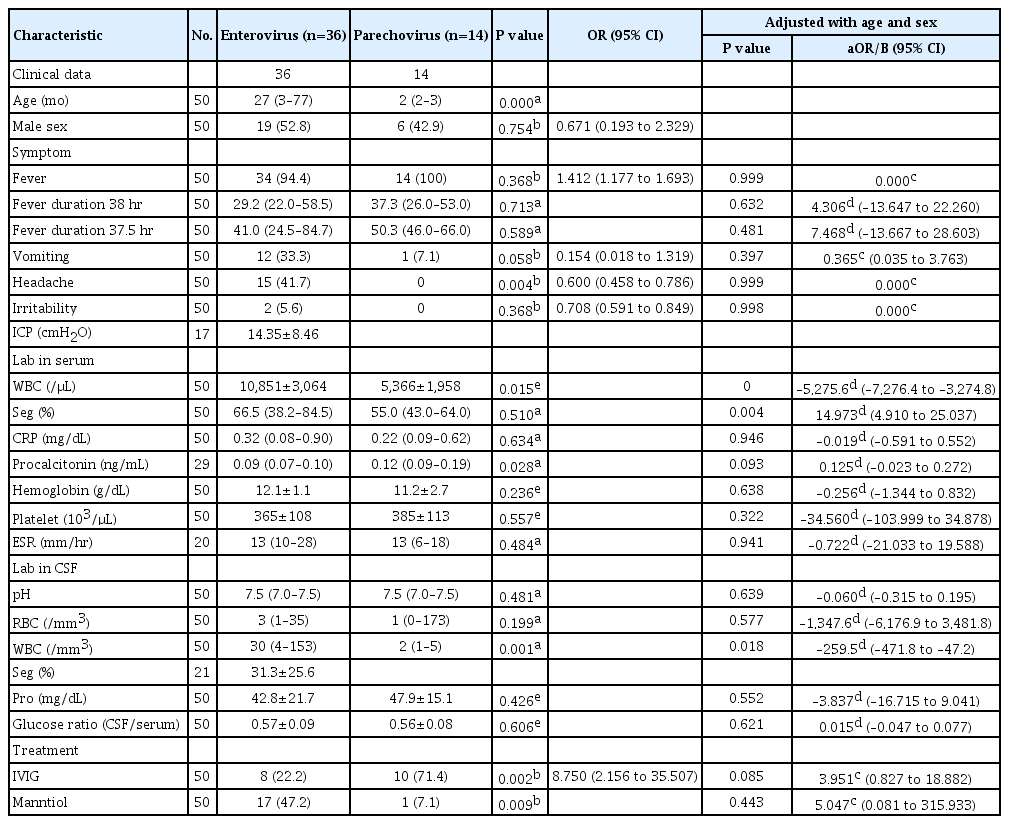
Samples from a total of 161 patients (males 96, females 65) were examined using film array-ME and 50 patients were positive for EV or HPeV infections (Fig. 1). Among the 50 patients, 36 were positive for EV (one also had rotavirus-positive stool) and 14 were positive for HPeV (one was also positive for the varicella-zoster virus). The proportion of males with EV and HPeV infections were 19 of 36 (52.8%) and six of 14 (42.9%), respectively (P=0.671). Patients with EV infection were older than those with HPeV infection (median 27 [interquartile range, IQR, 3 to 77] and 2 months [IQR, 2 to 3], respectively; P=0.000) (Table 1).
2. Clinical symptoms
There was no significant difference in the total fever duration between the EV and HPeV group (29.2 [IQR, 22.0 to 58.5] and 37.3 hours [IQR, 26.0 to 5.0], respectively; P=0.713). Headache and vomiting were more prevalent in the EV group (P=0.004 and P=0.058) while there was no significant between-group difference in irritability (P=0.708). After adjusting for age and sex, there were no significant between-group differences in symptoms such as headache, vomiting, duration of fever, and irritability (P=0.999, P=0.397, P=0.632, and P=0.998, respectively).
3. Laboratory findings
The serum WBC count was higher in the EV group than in the HPeV group (10,851±3,064 vs. 5,366±31,953, P=0.015). The CSF study was conducted within 24 hours of admission which means that the CSF study was performed within 2 to 3 days of symptom (fever) onset (totally 0 [IQR, 0 to 0.5]; EV 0 [IQR, 0 to 1]; HPeV 0 [IQR, 0 to 0]; P=0.068). There were no significant between-group differences in the segmented cell dominancy, CRP, and ESR (P=0.510, P=0.634, and P=0.484, respectively); however, procalcitonin was higher in HPeV (EV 0.09 [IQR, 0.07 to 0.10] vs. HPeV 0.12 [IQR, 0.09 to 0.19], P=0.028). The CSF WBC count was higher in the EV group than in the HPeV group (30 [4 to 153] vs. 2 [IQR, 1 to 5], P=0.001); however, there were no significant between-group differences in the CSF RBC, glucose, and protein levels. After adjusting for age and sex, the CSF and blood WBC count was lower in the HPeV group than in the EV group (CSF WBC count –259.53 [IQR, –471.8 to –47.2], P=0.018; WBC count in blood –5,275.6 [IQR, –7,276.4 to –3,274.8], P=0.000).
4. Treatment
Regarding treatment, the use of hyperosmotic fluid (mannitol) was higher in the EV group (P=0.009) while the use of immunoglobulin was higher in the HPeV group (P=0.002). Immunoglobulin was administered to 18 patients while the number of children treated with different intravenous immunoglobulin (IVIG) doses was as follows: four with 0.5 g/kg, seven with 1 g/kg, three with 1.5 g/kg, and four with 2.0 g/kg. After adjusting for age and sex, there was no significant between-group difference in the frequency of usage of mannitol and immunoglobulin.
5. Neurologic symptoms and follow-up
One newborn (female, 40 days old) who tested positive for HPeV had seizures with unilateral semiology, with an electroencephalograph showing a negative sharp wave. Her CSF WBC count was only 2/mm3, and multifocal small hyperintensities were observed using diffusion-weighted magnetic resonance imaging (MRI) of the bilateral cerebral white matter (Fig. 2A and B). A week after IVIG management, follow-up MRI indicated improvement in this newborn with unilateral seizure. The anticonvulsive drug (phenobarbital) dose was gradually reduced after which she showed no symptoms. Moreover, all the patients showed normal development without symptoms at the 12-month follow-up. A 46-day-old female with EV infection had fever, and CSF WBC count was 1,780/mm3 (Fig. 2C and D). She also underwent brain MRI and had a small hyperintense region in white matter on diffusion weighted MRI. She was administered with IVIG and improvements in her symptoms were noted. Until the age of 8 months, she showed normal development. Another 19-month-old male with simple febrile seizures tested positive for EV and the CSF WBC count was 1/mm3; there were no further neurologic symptoms. Lastly, a 55-month-old male who tested positive for EV presented with ataxic gait without fever. He had no vomiting or headache and a CSF WBC count of 23/mm3. Brain MRI was normal and spine MRI, which was performed after symptom improvement, was unremarkable. No relapse occurred during the 8-month follow-up period.
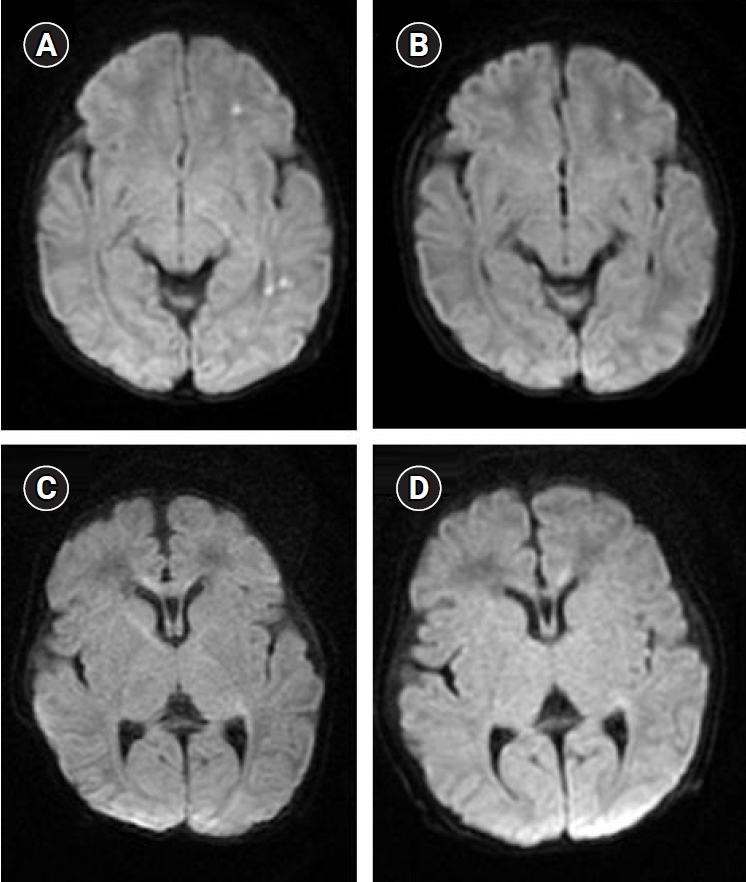
Magnetic resonance imaging scans of severe cases. (A) A 40-day-old female who had a fever with unilateral seizure tested positive for human parechovirus after CSF assessment. Multifocal small hyperintense lesions were transiently in the diffusion-weighted image of the bilateral cerebral white matter (including the corpus callosum splenium and in another image level and the bilateral perirolandic area and ventrolateral thalami in a later assessment) observable. (B) After 6 days, a follow-up study showed a slightly decreased signal with a small remnant. (C) A 46-day-old female who showed fever and was enterovirus-infected. A tiny, subtle high signal intensity was transiently in the diffusion-weighted image of the right side corpus callosum genu, observable. (D) After 6 days, an improved signal change was observed in the right corpus callosum genu. CSF, cerebrospinal fluid.
6. Image study and further neurologic study
Computed tomography (CT) scans were done in 18 EV-infected patients, five patients (three EV-infected, two HPeV-infected) underwent MRI, and three HPeV-infected patients underwent cranial ultrasonography. A total of 25 patients did not undergo brain imaging or electrophysiologic studies. Among those who underwent CT and cranial ultrasonography, none showed gross abnormalities, and a baby showed mild periventricular leukomalacia. Among the five patients who underwent MRI examination, two showed abnormal findings of high signal intensity on diffusion-weighted magnetic resonance images in the white matter of a relatively short duration that was fully reversible (Fig. 2).
Discussion
Both EVs and HPeVs belong to same viridae [6], have similar clinical aspects, and are common pathogenic causes of aseptic meningitis in young children, as observed in this study. Despite their very similar clinical characteristics, we found that HPeV is more common among neonates and that patients with HPeV had a lower CSF and blood WBC count even after adjusting for age and sex. Previous studies have reported clinical [19], neuroimaging [12], and prognostic [6,11], similarities between both viruses. Moreover, there has been a reported male predominance; however, it was not statistically significant [7]. In this study, we could not find differences in the clinical characteristics of HPeV and EV infections, such as headache, vomiting, and irritability.
We found that the CSF WBC count was relatively low in the HPeV group compared with the EV group. Previous studies also reported a low CSF WBC in this group [5,7,20]; however, other studies have not reported this finding [5,9,21]. This result means that patient with lower CSF WBC counts without HPeV assessment were not considered to have meningeal infection, and many of the fever cases with an unknown focus might have possibly resulted from HPeV infection. This is especially the case during summer and early fall [2], where aseptic meningitis caused by HPeV infection might lead to an increase in the cases of febrile children with mild headache. Moreover, there have been reports of infants with unknown fever focuses that were later determined to be caused by this virus [15]. Nearly absent pleocytosis in the CSF, which was previously considered as negative indicator of meningitis, was found in HPeV-infected patients [5,19]. This means that viral meningitis should be suspected even if the WBC count is less than 5 cells/mm3.
The age distribution, specifically the younger age preference of these viruses, as shown in this study, are possibly due to an actual age preference of the HPeVs [14,22] or selection bias due to the relatively mild symptoms in older children. Patients with low immunity are susceptible to viral infection, especially with rotaviruses; however, the presence of an intact immune system might deter the process of viral infection. In addition, many patients lack access to or prefer not to undergo lumbar puncture, which can cause selection bias regarding CSF examination, especially given that relatively mild symptoms of aseptic meningitis cannot be detected. Moreover, due to the fecal-to-oral route of infection of this virus, an infant’s siblings could also be infected by this virus. Using non-invasive assessment techniques, such as stool or nasal swab, can correct some of the selection bias.
Treatment of infants with IVIG may prevent brain viral infection from becoming fatal or severe sequelae [23]. Early administration of IVIG has been reported to improve fatal EV infections such as hepatitis with coagulopathy and thrombocytopenia or myocarditis [24]. Abzug et al. [17] also demonstrated that neutralization of EVs using 750 mg/kg IVIG can change the course of encephalitis with rapid cessation of viremia and can improve the prognosis. Moreover, other studies [12,15,19,25,26] have also reported the use of IVIG for prevention of possibly fatal cases of neonatal HPeV infection. Further studies are required on the use of IVIG in the treatment of HPeV infection to improve the infantile HPeV/EV management protocol. A few patients with HPeV infections also presented with white matter injury and the infection was transiently observed with a benign course; however, previous studies have reported neonatal HPeV infections with a fatal course [11,12,19]. We found less serious pathogenicity of HPeV compared to that reported by a previous study [12], which might be attributed to the use of IVIG therapy. The fatality of neonatal viral infection may be due to viral subdivision, which was not studied in this study. Moreover, it might be influenced by the use of IV globulin in the early disease course. However studies with long-term follow-up are needed to confirm this.
This study has several limitations. As previously mentioned above, older children with mild symptoms did not undergo lumbar puncture, which possibly resulted in selection bias. An early CSF study can show a low WBC count, which then increased during the subsequent few days. Moreover, we did not perform MRI examinations on babies with neurologic symptoms or CT and cranial ultrasonography in children/babies with mild symptoms; therefore, we might have missed cases with white matter injury. Diffusion-weighted MRI image can detect white matter injury resulting from HPeV encephalitis [12]. In addition, we only conducted this study for a period of one season in one year; therefore, there is a need for studies with more participants and a longer study period [10,18,21]. Moreover, we could not determine the subtype classifications of the EVs and HPeVs [27], which is important given the different fatalities of each subclass of the viruses [4]. Lastly, since we only used one study method to assess the samples, there is a possibility of false positives and negatives.
We found that HPeV and EV infection have similar clinical characteristics and mostly similar laboratory values. However, we found between-group differences in the predominant age and CSF and blood WBC count with the CSF WBC count in HPeV patients being less than the cut-off value for aseptic meningitis. Therefore, we should consider assessing for viral infection even when the CSF WBC count is low. Lastly, MRI examination can help in the positive diagnosis of EV and HPeV infections, especially in infants.
Notes
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SR. Data curation: SR. Formal analysis: SR. Funding acquisition: SR. Methodology: SR. Project administration: SR. Visualization: SR. Writing-original draft: SR. Writing-review & editing: SR.