Neurological Symptoms of SARS-CoV-2 Infection in Pediatric Patients
Article information
Abstract
Purpose
Coronavirus disease 2019 (COVID-19) causes various neurological symptoms in children, as well as respiratory symptoms, and the number of reported cases is increasing with the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. This study aimed to investigate the neurological symptoms and incidence in pediatric patients hospitalized with COVID-19.
Methods
We retrospectively analyzed the medical records of patients under the age of 18 diagnosed with COVID-19 and admitted to National Health Insurance Service Ilsan Hospital using real-time reverse transcription–polymerase chain reaction from December 2020 to March 2022. We reviewed data on the age of confirmed COVID-19 patients, fever, and respiratory, gastrointestinal, and neurological symptoms. We evaluated the chief complaints of hospitalization and classified them as non-neurological or neurological, according to the chief complaints that caused the most discomfort.
Results
Among 376 patients, 63 (16.8%) and 313 (83.2%) patients were classified as having neurological and non-neurological symptoms, respectively. The most common neurological symptoms were headache (49, 13.0%), followed by seizures (39, 10.4%), myalgia (24, 6.4%), and dizziness (14, 3.7%). Additionally, there were patients with anosmia (nine, 2.4%), ageusia (four, 1.1%), and visual disturbance (two, 0.5%). Of the 39 patients who experienced seizures, 15 (15/39, 51.7%) had no symptoms except fever, and seizures were the only main presenting symptom of SARS-CoV-2 infection.
Conclusion
Neurological symptoms are common in pediatric COVID-19 patients. Seizures can be an early symptom of SARS-CoV-2 infection and should not be underestimated during the COVID-19 pandemic.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has infected more than 500 million people worldwide. By May 2022, more than 17 million patients had been reported in the Republic of Korea, with children comprising 25.3% of reported cases [1].
Previous studies in adults have reported that COVID-19, which mainly shows respiratory symptoms, can also cause neurological symptoms. Neurological symptoms, which have mainly been reported in adults, range from headache and dizziness to seizures, and the neurological manifestations of COVID-19 range from neuropathy to stroke and encephalopathy [2]. In a study of 873 adults, the most common non-neurological symptoms were fever and cough, and common neurological symptoms included myalgia, headache, and dizziness. More than 60% of patients had hyposmia and dysgeusia, and these symptoms improved approximately 8 weeks later [3]. In another adult study, of 6,147 COVID-19 patients, 110 died and five had seizures (seizure rate, 0.08%) [4].
Many studies have been conducted to obtain more information on the clinical characteristics of COVID-19; however, most of them were for adults, and studies on children remain limited [5]. Most studies on pediatric patients have focused on respiratory symptoms and multisystem inflammatory syndrome in children (MIS-C); however, several studies on neurological manifestations in children have been reported [2]. There have been cases of pediatric patients with various clinical manifestations, from Guillain-Barre syndrome to sensor and motor polyradiculopathy, epileptic seizures, encephalopathy, and abnormal eye movements [6,7]. In a study of 90 pediatric patients with COVID-19, 13 (14.4%) showed neurological symptoms, which ranged from mild (headache, muscle weakness, anosmia, ageusia) to severe (status epilepticus, encephalopathy, and demyelinating events) [8].
After an 11-year-old boy in the United States visited the hospital for a new-onset seizure and was diagnosed with COVID-19 [9], cases of seizures have been reported in pediatric patients infected with SARS-CoV-2 [10]. Therefore, unlike in adults, it has been suggested that seizures may be a major sign of acute COVID-19 in children [11]. In particular, after the emergence of the Omicron (B.1.1.529) variant in South Africa, the increased infection rate was consistent with a rapid increase in COVID-19-related hospitalizations in children, and 19 out of 61 patients showed seizures [12]. Therefore, there is a need for further research on the clinical symptoms of pediatric patients in Korea, where the Omicron variant is prevalent.
This study aimed to investigate the neurological symptoms and incidence compared with non-neurological symptoms in pediatric patients hospitalized with COVID-19 at a single center in Korea. We also evaluated the age, presence of fever, and severity of seizures in patients infected with COVID-19 in the context of other neurological symptoms.
Materials and Methods
1. Study population
This retrospective study was performed at the National Health Insurance Service Ilsan Hospital from December 2020 to March 2022. Patients aged <18 years diagnosed with COVID-19 were included in the study. A sterile nasopharyngeal swab was inserted into the patient’s nasopharynx. COVID-19 was confirmed by detecting SARS-CoV-2 RNA in the nasopharyngeal swabs using real-time reverse transcription–polymerase chain reaction (RT-PCR). RT-PCR for SARS-CoV-2 detection was conducted at various hospitals or the Research Institute of Public Health using COVID-19 test kits. The inclusion criteria were as follows: (1) patients diagnosed with COVID-19 and (2) patients admitted to the National Health Insurance Service Ilsan Hospital. The exclusion criteria were as follows: (1) confirmed other respiratory infections and (2) voluntary discharge before the end of the isolation period. This study was approved by the Institutional Review Board of the National Health Insurance Service Ilsan Hospital (IRB 2022-03-016). Written informed consent by the patients was waived due to a retrospective nature of our study.
2. Data collection
We retrospectively reviewed the patients’ medical records and collected data on the age upon COVID-19 confirmation, the duration of hospitalization, the presence of underlying diseases, vaccination history, fever, respiratory symptoms, gastrointestinal symptoms, neurological symptoms, chest X-ray findings, brain magnetic resonance imaging (MRI) findings, laboratory findings, and electroencephalography (EEG) findings.
First, we evaluated patients’ chief complaints during hospitalization. Children infected with COVID-19 were hospitalized through the emergency room at the hospital or entered the Residential Treatment Center and then admitted to the National Health Insurance Service Ilsan Hospital if their symptoms worsened. When a patient was hospitalized through the Residential Treatment Center, symptoms requiring hospitalization were evaluated as the chief complaint rather than the first symptom. We divided patients into non-neurological and neurological groups according to the chief complaint that caused the most discomfort. Chief complaints such as headache, dizziness, seizure, and anosmia were classified as neurological, and asymptomatic, fever-only, respiratory symptoms, gastrointestinal symptoms, chest pain, and rash were classified as non-neurological. In addition to the chief complaint, new symptoms that appeared after hospitalization were also recorded daily. If the patient complained of both non-neurological and neurological symptoms, they were classified according to which symptoms were most uncomfortable during hospitalization.
Based on the International Classification of Headache Disorders, headache was evaluated to be related to COVID-19 if it met the criteria for “headache attributed to systemic viral infection” [13]. A patient with a headache who was hospitalized because of a high fever was classified into the non-neurological group, whereas a patient with febrile seizure was classified into the neurological group. In addition to the chief complaints, we evaluated multiple respiratory, gastrointestinal, and neurological symptoms of each individual. Underlying diseases, including prematurity and epilepsy, were evaluated. For allergic rhinitis and asthma, only patients currently taking drugs were included.
The onset date was based on the date of RT-PCR examination; the number of patients was evaluated monthly, and the number of patients who had seizures in the neurological group was evaluated separately. EEG and brain MRI were performed on the day of discharge when the patient was released from isolation if the patient’s condition was not status epilepticus.
3. Statistical analysis
Statistical analyses were performed using MedCalc version 19.2 (MedCalc Software bvba, Ostend, Belgium). Variables with a normal distribution were represented as mean and standard deviation (SD), while variables without a normal distribution were represented as medians and interquartile ranges. For comparisons of the two groups, the independent t-test or Mann-Whitney U test was used for continuous variables, and the chi-square test or Fisher exact test was used for categorical variables. All tests were considered statistically significant when P values were less than 0.05.
Results
1. Baseline characteristics
Between December 2020 and March 2022, 380 patients were diagnosed with COVID-19 and admitted to the National Health Insurance Service Ilsan Hospital. Four patients who were voluntarily discharged owing to the COVID-19 ward environment were excluded from the study. A total of 376 patients were enrolled in this study.
The mean±SD age at diagnosis of COVID-19 for all patients was 6.0±4.7 years and 218 patients (58.0%) were male. Patients aged 5 to 12 were hospitalized more frequently (n=159; 42.3%) than other age groups, and the mean hospitalization period was 8.4±2.3 days. Most patients were not vaccinated against COVID-19 (98.9%) (Table 1).
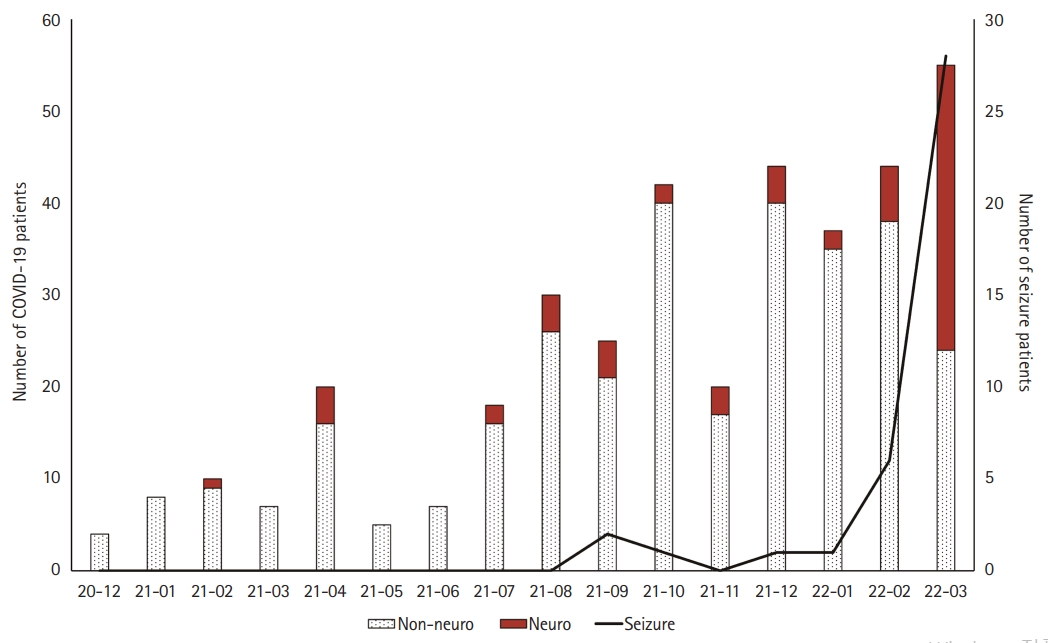
We evaluated the chief complaints during patients’ hospitalization. There were 313 patients in the non-neurological group (83.2%) and 63 patients in the neurological group (16.8%). There was no significant difference between the two groups in baseline characteristics, except for the proportion of patients under the age of 1 year and the hospitalization period. There was no significant difference in the ratio of obesity, defined as a body mass index of 25 kg/m2 or higher, between the two groups. The number of patients per month tended to increase over time, and 242 patients were hospitalized between October 2021 and March 2022 (64.4%). In March 2022, 31 patients in the neurological group were hospitalized, which was the highest number among all months (31/63, 49.2%) (Fig. 1).
2. Non-neurological symptoms: overall and comparison between the groups
Twenty-nine patients were asymptomatic from before hospitalization to discharge, accounting for 7.7%. Of the total patients, 86 patients (22.9%) had no fever. There were 85 patients (22.6%) with a high fever above 39.0°C, and high fever was more frequent in the neurological group (n=23, 36.5%) (P=0.004). The most common non-neurological symptom in both groups was fever (n=290, 77.1%), followed by cough, rhinorrhea, and sore throat. However, cough (65.8%) and rhinorrhea (31.3%) were significantly more common in the non-neurological group than in the neurological group (36.5%, P<0.001; 17.5%, P<0.027, respectively). Of the total patients, 67 (17.8%) showed symptoms of the gastrointestinal tract, and these symptoms often appeared a few days after hospitalization, rather than on the first day of hospitalization. The most common gastrointestinal symptom was diarrhea (9.6%), followed by vomiting (7.4%) and abdominal pain (5.6%). There was no significant difference in gastrointestinal symptoms between the two groups (P=0.522). Twenty-two patients showed irregular erythematous rashes, which usually appeared throughout the body approximately 5 days after hospitalization. Twenty-eight patients were diagnosed with pneumonia, five with acute bronchiolitis, three with croup, and one with MIS-C (Table 2).
3. Neurological symptoms: overall and comparison between the groups
The most common neurological symptom among all patients was headache (n=49, 13.0%), followed by seizures (n=39, 10.4%), myalgia (n=24, 6.4%), and dizziness (n=14, 3.7%). However, the most common symptom in the neurological group was seizures (39/63, 61.9%) (Table 3). In the non-neurological group, 35 (11.2%) and nine (2.9%) patients had symptoms of headache and dizziness, respectively. Most patients did not present with neurological symptoms.
Nine patients (14.3%) had loss of smell, and four patients (6.3%) had loss of taste. Anosmia and ageusia only mildly improved until discharge, and did not completely resolve. Four patients (6.3%) had rhabdomyolysis, with creatine kinase levels increasing by more than 400 IU/L, and no patient had worsening symptoms after intravenous hydration. Patients with cerebellar ataxia had normal brain MRI findings; however, the symptoms persisted. Following oral administration of methylprednisolone for 7 days, the symptoms improved. A 16-year-old female patient suddenly developed left homonymous hemianopsia on the seventh day of hospitalization, and posterior cerebral artery infarction was confirmed on brain MRI. She recovered from visual loss 5 days after taking aspirin.
4. Seizures as the main presenting characteristics of COVID-19
The most common symptom in the neurological group was seizures (39 patients). Two of these patients did not have a fever. Fifteen patients (15/39, 51.7%) had no symptoms other than fever throughout hospitalization, and seizures were the only main presenting symptoms of COVID-19.
Ten patients (10/39, 25.6%) had a history of febrile seizures, and three patients with epilepsy had Dravet syndrome, Landau-Kleffner syndrome, and focal epilepsy. There were 15 patients (15/39, 38.5%) aged >7, four of whom had no previous history of febrile seizures (10.2%). Most of the seizures were generalized tonic-clonic seizures (n=29, 74.4%); however, 10 patients showed focal impaired-awareness seizures (10/39, 25.6%). Of the 10 patients who had focal seizures, six patients underwent EEG and four showed abnormal findings (respectively, left hemisphere slowing, right central and parietal slowing, bilateral frontal slowing, and left occipital sharp wave discharges). Of the 29 patients who showed generalized seizures, 13 patients underwent EEG, with normal findings except for one who showed generalized spike and wave discharges. Three patients with focal seizures underwent brain MRI; one showed left ventriculomegaly, while another showed developmental venous anomalies in right occipital area. Thirty-five patients (35/39, 89.7%) experienced a seizure on the first day of fever, and there were no cases of seizures on the second day of hospitalization (Table 4). Since January 2022, when the Omicron variant became predominant in Korea, the number of patients with seizures has increased rapidly (Fig. 1).
Discussion
This study investigated various neurological symptoms and the incidence rates thereof in pediatric patients hospitalized with COVID-19. The most common neurological symptoms among all patients was headache (13.0%), followed by seizures, myalgia, and dizziness. The most common symptom in the neurological group was seizures (39/63, 61.9%), followed by headache and anosmia. Seizures were the only major presenting symptoms of SARS-CoV-2 infection, and more than half of the patients with seizures had no symptoms other than fever. In this study, the proportion of patients under the age of 1 was higher in the non-neurological group. It is possible that fewer patients in this age group were hospitalized because they could not express subjective expressions such as headache, dizziness, anosmia, and ageusia, whereas seizures could be more directly observed. In addition, the guardians of younger patients more frequently requested hospitalization, rather than treatment in a Residential Treatment Center, even if the patient had mild respiratory symptoms. The duration of symptoms before hospitalization was shorter in the neurological group, which seems to have been because patients with seizures and severe headaches were usually hospitalized on the same day. However, patients with fever and respiratory symptoms in the Residential Treatment Center were hospitalized later if the symptoms did not improve.
In previous studies on COVID-19, children's symptoms were thought to be milder than those of adults, and pediatric symptoms tended to be underestimated because they had lower mortality rates than adults. However, the number of intensive care unit (ICU) hospitalizations has increased since MIS-C has been reported in children [14,15]. In our study, three patients were admitted to the ICU for respiratory difficulty; however, no patient was admitted to the ICU due to neurological symptoms. In another large cohort study, 1,278 children were diagnosed with acute SARS-CoV-2, of whom 215 were diagnosed with MIS-C. Forty-four percent of patients in that cohort showed neurological signs, and the most common neurological signs were headache (16%) and acute encephalopathy (15%) [16]. Headaches mainly showed a tension-type-like phenotype and were bifrontal, while 25% of patients had migraine-like characteristics [17]. Encephalopathy was defined as altered mental status and lethargy, with reports of MIS-C-related encephalopathy mainly in pediatric patients [2]. However, that study investigated neurological symptoms associated with MIS-C, whereas in our study, it was difficult to compare the proportion of neurological symptoms because only one case could be diagnosed as typical MIS-C. In another meta-analysis with 3,707 children, myalgia (14.3%), headache (3.7%), seizures (3.1%), and encephalopathy (12.6%) were the main neurological symptoms. Approximately 1% of children were reported as having definite neurological complications, with rare cases of intracranial hemorrhage, Guillain-Barre syndrome, and visual disturbance [18]. For comparison, in a multi-center study of 873 adults, the most common neurological symptoms were smell and taste dysfunction (64.3%), myalgia (24.8%), headache (12.6%), and dizziness (11.9%). In rare cases, there were patients with cerebrovascular events (n=10), demyelinating myelitis (n=1), and Guillain-Barre syndrome (n=1) [3]. Seizures and encephalopathy were common in children, whereas loss of smell and taste were common in adults. However, it is possible that the frequency of anosmia and ageusia was underestimated because children cannot express these symptoms. In our study, 13% of patients showed headaches; 6.4% had myalgia, which was similar to a previous study; and 39 patients had seizures, more than in the previous study. In particular, based on a study reporting that headache attacks in pediatric patients with migraines occur more frequently during the COVID-19 pandemic, the association between COVID-19 and headaches is thought to be significant [19]. In this study, there were no cases of facial paralysis, but a previous study reported some cases of facial paralysis as a neurological symptom of COVID-19 [20]. In addition, as in the previously reported case of cerebellar ataxia, our patient responded well to methylprednisolone treatment [21]. However, in our study, only a few patients lost their senses of taste and smell. This finding should be interpreted in light of the fact that our cohort had 46.8% of patients under the age of 5, for whom it was difficult to identify these symptoms [22].
In our study, 15 patients had seizures without respiratory symptoms, and in previous studies, new neurological symptoms, including the central and peripheral nervous systems, were present even in the absence of respiratory symptoms [23]. It is now well known that SARS-CoV-2 infection exacerbates epileptic seizures in children [24]. However, in our study, two epilepsy patients showed status epilepticus and responded well to antiepileptic drugs. In particular, the rapid increase in seizure cases since January 2022, when the Omicron variant became predominant in Korea, is thought to be correlated with the Omicron variant.
The mechanism of SARS-CoV-2 infection was initially known to involve cell entry through angiotensin-converting enzyme 2 (ACE2), thereby causing vascular damage, such as Kawasaki disease, as well as damage to the lung. SARS-CoV-2 has also been found to cause specific neurological symptoms such as seizures, headaches, anosmia, and ageusia, and these symptoms seem to occur in patients without neurological disorders. Several pathological hypotheses have been proposed after it was found that specific neurological symptoms may occur due to the effects of ACE2 and the central nervous system on the renin-angiotensin-aldosterone system [25]. Currently, four major hypotheses have been proposed to explain the neurological symptoms of SARS-CoV-2 infection: (1) direct injury to neural cells; (2) vascular endothelial injury; (3) post-infectious inflammation; and (4) para-infectious inflammation [2,24]. The virus is known to directly invade the olfactory nerve and affect the central nervous system through the cribriform plate [22,26]. Endothelial damage can trigger thrombotic events such as stroke, as well as SARS-CoV-2 release. In addition, post-infectious and para-infectious inflammation can cause specific neurological symptoms through innate and adaptive immune activation. These excessive inflammatory reactions are thought to cause neurological symptoms such as headaches, seizures, central nerve palsy, and encephalopathy [27]. Therefore, children infected with SARS-CoV-2 frequently present with neurological symptoms, and pediatric patients experienced more epileptic seizures during the period of the pandemic characterized by the predominance of the Omicron variant, which mainly caused symptoms of upper respiratory tract infections [28].
The present study had several limitations. First, since there were many young patients under the age of 5 years, the frequency of neurological symptoms such as anosmia and ageusia may have been underestimated. Second, due to the need for negative pressure facilities for COVID-19, for most patients, EEG and brain MRI were not performed on the first day of hospitalization. Third, since the isolation period for COVID-19 has been reduced from 10 days to 7 days in Korea, it is possible that the change in the isolation policy, rather than the severity of the disease, affected the hospitalization duration.
In conclusion, our results identified the neurological symptoms and incidence of pediatric patients hospitalized with SARS-CoV-2 infection. Common neurological symptoms were headache, seizures, myalgia, and dizziness. In addition, neurological symptoms of anosmia, ageusia, ataxia, and visual disturbance were also observed in a small number of patients. We suggest that seizures may be an early symptom of SARS-CoV-2 infection and should not be underestimated during the COVID-19 pandemic.
Notes
Hoon-Chul Kang is an associate editor of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: HCK. Data curation: DY. Formal analysis: DY and HCK. Methodology: HCK. Project administration: HCK. Visualization: DY. Writing-original draft: DY. Writing-review & editing: DY and HCK.
Acknowledgements
This study was supported by the National Health Insurance Service Ilsan Hospital grant (NHIMC2022CR038).