A SERPINC1 Mutation in a Patient with Cerebral Venous Thrombosis and Upper-Extremity Deep Vein Thrombosis
Article information
Cerebral venous thrombosis (CVT) in children is rare, with an incidence of 0.67 per 100,000 children, including newborns [1]. Its clinical manifestations may vary, including seizures, papilledema, headache, loss of consciousness, coma, and local neurological defects. The most common manifestation in neonates is seizures, whereas headache predominates in non-neonatal age groups [2]. Hereditary thrombophilia causes 34% to 41% of CVT cases, within which antithrombin (AT) deficiency is rarely reported [3]. Here, we report a case of CVT, pulmonary thromboembolism (PTE), and upper-extremity deep vein thrombosis (DVT) associated with AT deficiency due to a novel missense mutation in SERPINC1.
A 16-year-old boy visited our emergency department with a 5-day history of headache, nausea, and vomiting. The patient's height and weight were 178 cm and 90 kg, respectively. His body mass index was 28.4 kg/m2, indicating obesity. He had a history of taking psychiatric medication for intermittent explosive disorder at a private hospital 1 year ago. Without his parents, the family history of CVT was unknown. On admission, the patient was conscious and no specific findings were noted. His vital signs were as follows: blood pressure, 121/70 mm Hg; pulse, 72 beats/min; respiratory rate, 20 breaths/min; body temperature, 36.3°C. A neurological examination revealed cervical rigidity. The hemoglobin level (15.6 g/dL), white blood cell count (12,640/mL), platelet count (280,000/mL), prothrombin time (12.3 seconds), and activated partial thromboplastin time (23.9 seconds) were normal. Cerebrospinal fluid analysis showed 12 red blood cells/mm3 and 8 white blood cells/mm3, with protein and glucose concentrations of 58 and 73 mg/dL, respectively, and an opening pressure of 38 mm Hg. An ophthalmologic examination for papilledema was not performed. Brain magnetic resonance imaging demonstrated bilateral thalamic infarction and multifocal petechial hemorrhages around the bilateral cortical veins, while brain magnetic resonance venography (MRV) detected right transverse/sigmoid sinus thrombosis and superior/inferior sagittal sinus thrombosis (Fig. 1A and B). Chest computed tomography with contrast revealed thrombi in the jugular vein, subclavian vein, brachiocephalic vein, and proximal superior vena cava on the right side and a filling defect in the distal portion of the main pulmonary trunk and bilateral segmental pulmonary arteries (Fig. 1C).
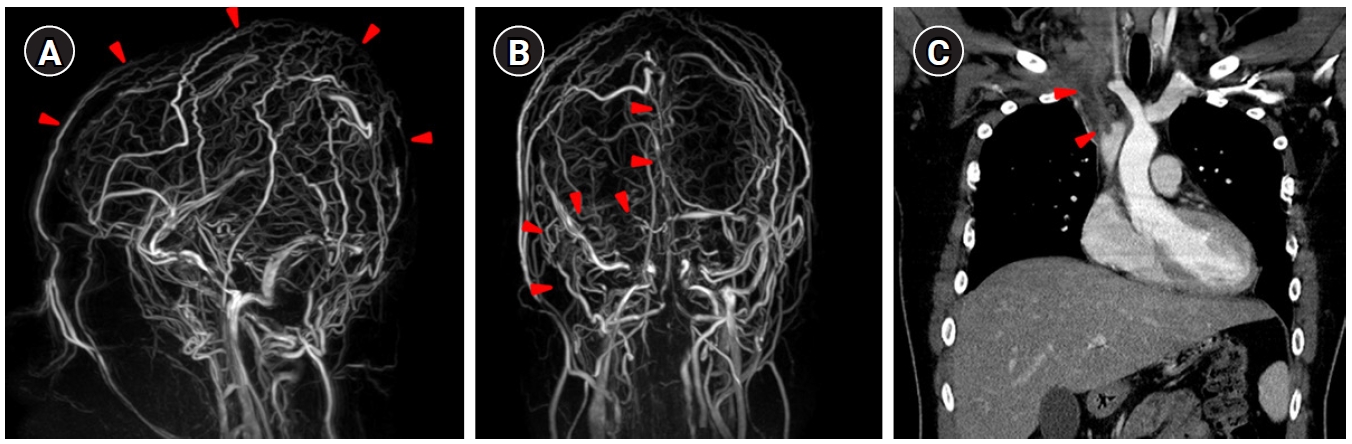
Extensive cerebral venous thrombosis and upper-extremity deep vein thrombosis. Contrast-enhanced magnetic resonance venography images of the lateral view (A) and posterior view (B) showed extensive filling defects along the superior/inferior sagittal sinuses and right transverse/sigmoid sinuses (arrowheads). (C) Chest computed tomography with contrast revealed thrombi in the jugular vein, subclavian vein, brachiocephalic vein, and proximal superior vena cava on the right side (arrowheads).
The autoimmune test results were negative, with the following results: factor VIII, 129.9% (60%–140%); factor IX, 151.2% (60%–140%); factor XI, 90% (60%–140%); protein C Ag, 131.07% (72%–160%); and protein S Ag, 123.71% (60%–150%). AT III was reduced to 62.2% (75%–125%). The amino acid and urine organic test results were negative. The. homocysteine level was elevated to 17.3 μmol/L (0 to 15), whereas ADAMTS13 activity was 83.5% (>40%). No methylenetetrahydrofolate reductase (MTHFR) A1298C or C677T polymorphisms were found. Additionally, no mutation in the JAK2V617F gene was detected. Gene panel testing for hereditary blood coagulation disorder was performed, and the patient was found to be heterozygous for a c.409-1G>T mutation in the SERPINC1 gene. The SERPINC1 gene is related to thrombophilia seven due to AT III deficiency (OMIM 613118, autosomal dominant or recessive), and a loss-of-function mutation of this gene is established as a mechanism of disease. The c.409-1G>T variant is a canonical -1 splice variant that is assumed to cause aberrant splicing and has not been detected in the general population (gnomAD, Korean Reference Genome Database [KRGDB]). The same variant has been reported in a family with AT deficiency [4], and the clinical phenotype and laboratory findings of our patient are consistent with AT deficiency. Although it was not possible to determine whether this mutation was inherited or de novo due to the lack of a parental test, this c.409-1G>T variant was interpreted as a pathogenic variant according to the American College of Medical Genetics and Genomics guideline (2015) on the basis of the above findings [5].
Starting on the first day of hospitalization, the patient was treated with subcutaneous low-molecular-weight heparin (1 mg/kg) for anticoagulation and osmotic agents (mannitol and hypertonic saline) for increased intracranial pressure. The patient’s headache improved on the 10th day of hospitalization. A direct oral anticoagulant (apixaban) was administered on the 25th day of hospitalization. Brain MRV on the 24th day after admission demonstrated resolution of almost all previously observed multifocal cerebral thrombi.
To our knowledge, this is the first case report in Korea to describe CVT, PTE, and extensive upper-extremity DVT associated with a SERPINC1 gene mutation. The glycoprotein, AT (58 kDa), belongs to the serine protease superfamily and is a potent inhibitor of many coagulation proteases, including thrombin and factor Xa. Deficiency of AT is a risk factor for venous thromboembolism and has a prevalence of 1% to 5% in patients with venous thrombosis and 0.2% in the general population [6]. Congenital AT deficiency is an uncommon autosomal dominant disorder, while acquired AT deficiency can be attributed to decreased AT production, increased protein consumption, or enhanced clearance [7]. The major clinical manifestations of AT III deficiency are young age at onset, idiopathic venous thrombosis, family history, recurrent venous thromboembolism, and thrombosis in an unusual site (cerebral or mesenteric veins) [8]. In our case, the patient had extensive idiopathic thrombosis, including CVT and DVT, but he had no typical risk factors of age of onset and thrombotic events, such as surgery, immobility, or trauma. In pediatric cases in Korea, one study reported venous thrombosis in the lower extremities caused by simultaneous AT III and protein S deficiencies, while another study reported neonatal cardiac thrombosis and CVT due to AT III deficiency [9,10]. In our patient, CVT, PTE, and upper extremity DVT were observed in the absence of specific risk factors, and these conditions were associated with AT deficiency that was genetically confirmed. Therefore, genetic testing should be considered even when there are no risk factors for patients with extensive thrombosis.
This case was reviewed and approved by the Institutional Review Board of Keimyung University Dongsan Hospital (IRB No. 2022-02-096). Informed consent was waived by the board.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JCB and JHH. Data curation: JCB and JHH. Formal analysis: JCB and JHH. Visualization: JHH. Writing-original draft: JCB and JHH. Writing-review & editing: JHH.
