Robot-Assisted Stereoelectroencephalography for Pediatric Epilepsy Surgery: The First Case in Korea
Article information
Stereoelectroencephalography (SEEG) was developed by Talairach and Bancaud in France for epilepsy surgery in the 1950s [1]. With the recent advent of new technologies, such as robot-assisted navigation and stereotactic image guidance, SEEG has been used widely throughout the world. While SEEG has gradually been accepted for treatment in adults, there is less consensus regarding its utility in children. A recent series included a substantial cohort of pediatric patients, suggesting that the enhanced epileptogenic zone (EZ) localization through SEEG may benefit both adult and pediatric populations [2]. Herein, we describe the first case of epilepsy surgery performed using robot-assisted SEEG in a child in Korea.
The KYMERO® robot-assisted SEEG system (Koh Young Technology, Seoul, Korea) developed for the management of intractable epilepsy (IRB approval No.: 1-2017-0059) was used for this patient. Written informed consent by the patients was waived due to a retrospective nature of our study. The authors have no conflicts of interest with the company that developed KYMERO®. In a female patient (patient 1 [P1]), seizures started at 8 years of age with vacant staring and sometimes evolved to clonic seizures on the right side of the face or tonic seizures on the right arm. Brain magnetic resonance imaging (MRI) showed a large, cavernous hemangioma on the left superior frontal gyrus (Fig. 1A). The hemangioma was resected, but the same seizures continued. After a year, P1 visited the authors’ clinic and had already been prescribed three antiepileptic drugs. Epileptiform discharges on P1’s interictal and ictal electroencephalography began with sharp wave discharges on the central vertex and both frontocentral electrodes (Fig. 1B and C). Due to P1’s bilateral epileptiform discharges, we re-operated using a robot-assisted SEEG procedure. Four electrodes were inserted into the left anterior, middle, and posterior superior frontal cortex, as well as the motor cortex. An additional electrode was inserted into the contralateral posterior superior frontal gyrus (Fig. 2A and B). There were seven episodes of habitual seizures; the seizure onset zone was consistently around the left posterior superior frontal gyrus, and independent epileptiform discharges were found on the left middle superior frontal gyrus during an interictal period (Fig. 2C). The seizure onset zone and the irritative zone were completely resected with intraoperative motor evoked potential monitoring, and electrocorticography confirmed normal brain activity on the resection margins (Fig. 2D). Since the procedure, P1 has been free of seizures for 8 weeks and has discontinued clobazam. The robot-assisted SEEG procedure is recommended for re-operation for bilateral epileptiform discharges. The procedure also accurately confirmed the ictal onset zone in this patient.
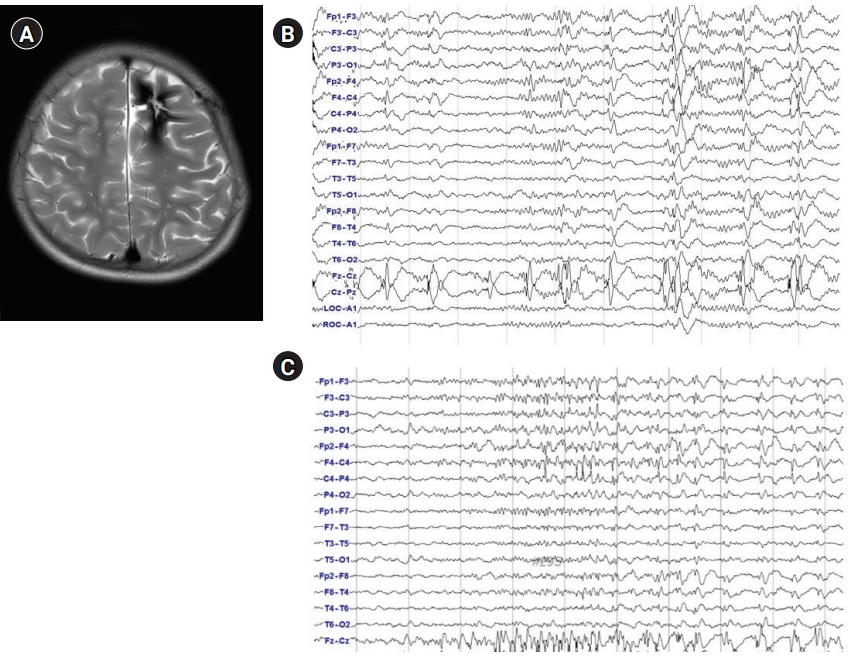
Phase I studies. (A) Brain magnetic resonance imaging, axial T2 sequence through the upper frontal lobe; (B) interictal scalp electroencephalography (EEG). Repetitive sharp and wave discharges on the central vertex and bilateral frontocentral electrodes; (C) ictal scalp EEG onset. Low-amplitude fast activities are seen on the central vertex and bilateral frontocentral electrodes.
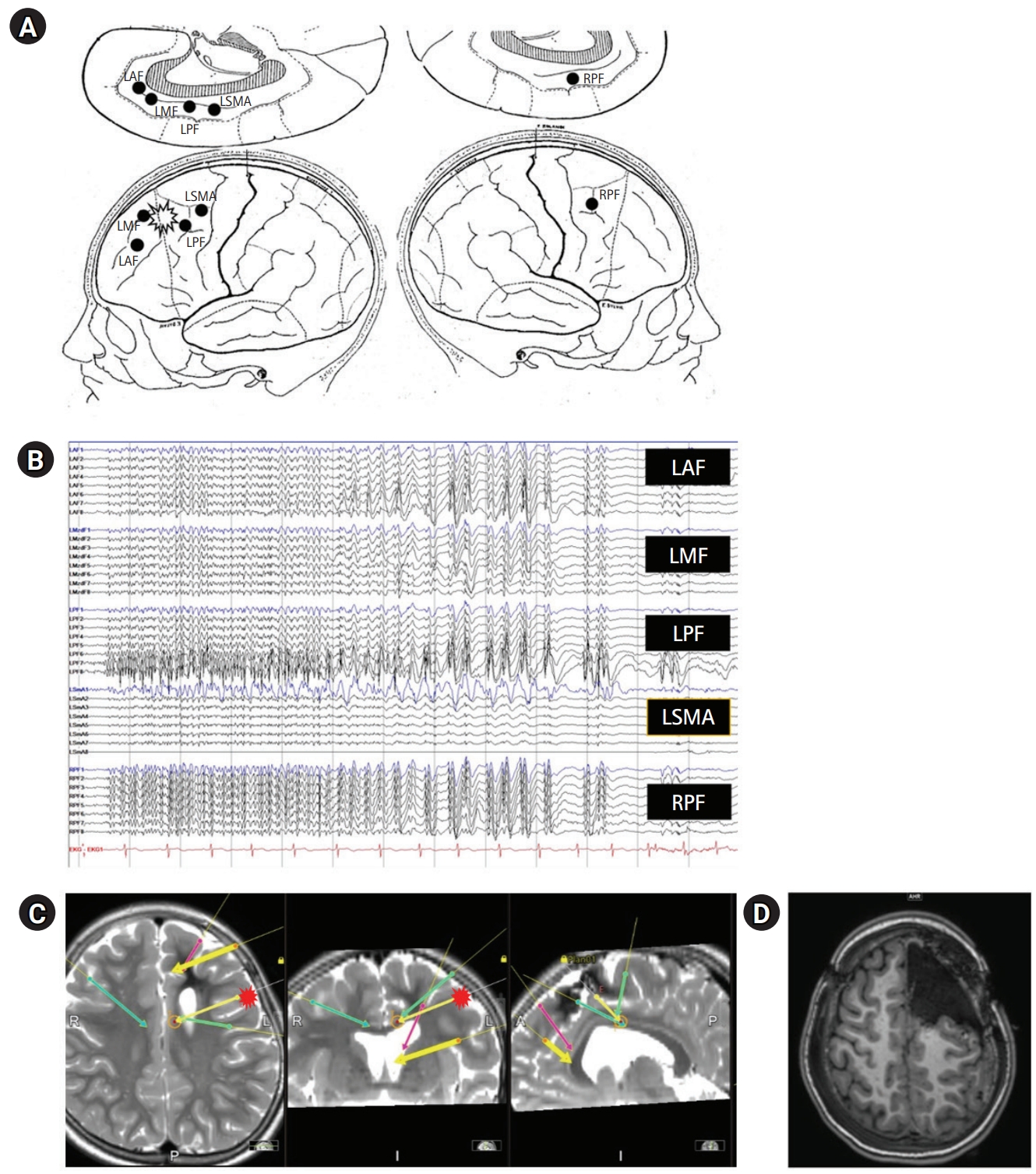
Phase II studies. (A) Stereoelectroencephalography (SEEG) scheme, with lateral and medial views on both hemispheres. (B) SEEG placement on brain magnetic resonance imaging (MRI). (C) SEEG ictal onset (fewer electrodes in a deeper location). Repetitive spiking signaling seizure onset is localized to channels on left posterior frontal cortex with nearly simultaneous spreading to contralateral hemisphere. (D) Postoperative brain MRI, axial T1 sequence through the upper frontal lobe. LAF (red), left anterior frontal; LMF (yellow, thick), left middle frontal; LPF (yellow, thin), left posterior frontal; LSMA (green, left), left supplementary motor area; RPF (green, right), right posterior frontal.
The Cleveland Clinic reported that EZ localization was achieved in 77% and 97% of subdural electrode and SEEG procedures, respectively, and that the degree of post-resection freedom from seizure was similar (68% and 70%, respectively) [3]. In that study, 31 out of 102 patients were pediatric patients, and it was suggested that improved EZ localization by SEEG could be helpful for children as well as adults. SEEG requires high-quality angiographic studies because avascular trajectory planning is a prerequisite for this procedure to be performed safely. We used computed tomography angiography and an MRI T1-weighted sequence with contrast enhancement. A meta-analysis showed that the risks of intracranial hemorrhage and surgical site infection are 4% and 2.3%, respectively, for subdural electrode placement and 1% and 0.8%, respectively, for SEEG [4]. There are few complications associated with long-term monitoring of SEEG, and up to 3 weeks of monitoring have been reported [5]. Sufficient bone thickness (≥2 mm) is required to fix anchor bolts for stable electrode placement for SEEG [4]. SEEG implantation can be performed safely in people ≥3 years of age.
SEEG is an effective diagnostic surgical tool for intractable epilepsy in children. Compared with the more commonly utilized subdural grids and strips, SEEG is superior for re-operation because it can identify deep, noncortical, and bilateral EZs. SEEG has been shown to be safe, with low rates of infection and hemorrhage. Given these advantages, SEEG has become a critical tool in the neurosurgeon’s armamentarium for pediatric epilepsy surgery. This emerging technology may improve perioperative outcomes and may be safer for pediatric patients with intractable epilepsy. However, large cohort and long-term follow-up studies are still needed.
Notes
Hoon-Chul Kang is an associate editor, Heung Dong Kim, Se Hee Kim and Joon Soo Lee are the editorial board members of the journal, but They was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: WSC, HDK, JWC, and HCK. Data curation: WSC and HCK. Formal analysis: HCK. Methodology: WSC, SHK, JSL, and HCK. Project administration: HCK. Visualization: HCK. Writing-original draft: KC and HCK. Writing-review & editing: HCK.
