Prognostic Factors of Patients with Postinfantile Epilepsy and Multiple Independent Spike Foci on Electroencephalography
Article information
Abstract
Purpose
Multiple independent spike foci (MISF) have been reported to be associated with hypsarrhythmia and slow spikes and waves. However, some patients with MISF demonstrate a good prognosis, such as benign focal epilepsy. This study aimed to elucidate the prognosis of epileptic children with MISF and to analyze the prognostic factors.
Methods
The subjects were 115 epileptic children aged 1 to 18 years who visited Pusan National University Children’s Hospital between November 2008 and July 2016 and in whom MISF were noted on electroencephalography. We excluded patients with infantile spasms, congenital metabolic diseases, neurodegenerative diseases, or post-encephalitic epilepsy. We retrospectively reviewed participants’ clinical information. Seizure control was defined as no seizures over 6 months at the last visit. Prognostic factors were analyzed in the seizure control (group A; 84 [73%]) and no seizure control (group B; 31 [27%]) groups.
Results
Generalized seizure (P=0.033), intellectual disability (P<0.001), cerebral palsy (P=0.046), and abnormal background activity and electrodecrements on electroencephalography (P<0.001) were significantly more common in group B. No clinically significant abnormalities were noted on magnetic resonance imaging of the brain. The MISF improved on follow-up electroencephalography in 58 (71.6%) patients in group A versus 10 (35.7%) in group B (P=0.002).
Conclusion
Despite MISF on electroencephalography, two-thirds of patients had a benign clinical course, particularly those with post-infantile epilepsy and no infantile spasms. The prognostic factors of poor outcomes were generalized seizures, intellectual disability, and abnormal background activity on electroencephalography.
Introduction
Historically, in the 1970s, the presence of multiple independent spike foci (MISF) on electroencephalography (EEG) was considered findings associated with hypsarrhythmia and slow spike and wave complexes, which were remarkable findings of infantile spasms and Lennox-Gaustaut syndrome (LGS) [1-3]. Blume [4] also described it was highly associated with intellectual abnormality despite a lack of infantile spasms or LGS. Thereafter, numerous studies on epileptic syndrome, infantile spasms, and LGS have led to their being widely accepted as epileptic syndromes distinctive from others; however, a few recent studies have aimed to reassess the significance of MISF in epilepsy.
Several recent studies proposed that patients for whom MISF are seen on EEG along with some characteristic clinical findings, who had poor prognosis and poor clinical course might be reclassified as having so-called severe epilepsy with MISF (SE-MISF) [5,6]. Nevertheless, it were not widely accepted yet and in the clinical setting, cases with unfavorable prognosis as well as favorable outcome despite MISF have been documented similar to those of patients with benign focal epilepsy. Here we aimed to analyze the prognosis of patients with post-infantile epilepsy with MISF and the prognostic factors associated with seizure control.
Materials and Methods
1. Patients and method
This retrospective study included patients aged 1 to 18 years who underwent an EEG study at the pediatric neurology clinic at Pusan National Children’s Hospital between November 1, 2008 and July 31, 2016. All subjects were patients with epilepsy diagnosed by the presence of two or more episodes of unprovoked seizures and MISF on EEG at least once before and during treatment. We included patients whose follow-up duration after MISF was over 1 year. We excluded patients who were diagnosed with infantile spasms and an expected poor prognosis; those who had a seizure onset before 1 year; or those with inborn errors of metabolism, neurodegenerative disease, acute encephalitis, or bacterial meningitis.
2. Method
This retrospective cohort study investigated the clinical information and factors associated with prognosis through medical chart reviews. MISF on EEG is defined as asynchronous multifocal epileptiform discharge from more than three foci with bilateral distribution and no adjacent electrical field. The treatment result was assessed as seizure control or non-control. The definition of seizure control was freedom from seizures for prolonged more than 6 months at the last visit by June 31, 2017. The patients with seizure control were included in the seizure control group (group A), while those without seizure control were in the no seizure control group (group B). The groups were compared to identify the prognostic factors influencing the therapeutic result.
Clinical information included sex, age at seizure onset, age at EEG study showing MISF, birth history such as gestational age and perinatal problems, and family history of epilepsy or febrile convulsions. The seizure profiles that were related to a history of seizures included seizure type, status epilepticus, duration between first seizure and start of anti-epileptic drug (AED) therapy, seizure frequency during the most recent 3 months from the identification of MISF on EEG. Comorbid conditions included intellectual disability (developmental delay in early childhood, including a speech-language delay), autism spectrum disorder, cerebral palsy, genetic disorder, and lesions suspiciously associated with epilepsy on magnetic resonance imaging (MRI). Other abnormal EEG findings were also analyzed, including abnormal background activities, focal slow waves, and generalized epileptiform discharges. We reviewed follow-up EEG study, which were most recent study within 1 year from last visit and compared with clinical outcome, seizure control.
Data from statistical analysis are expressed as medians and interquartile ranges (IQRs) for continuous and ordinal variables, and as counts and percentages for categorical variables. The two groups were compared using the chi-square test or Fisher’s exact tests for categorical and ordinal data, or the Mann-Whitney U test for non-parametric continuous data. A P<0.05 was considered significant. R Statistics and Microsoft Excel was used for statistical analysis.
3. Ethical statement
This study protocol was approved by the Institutional Review Board of Yangsan Pusan National University Hospital (no. 05-2019-047). The need for informed consent was waived by the board.
Results
1. Demographics
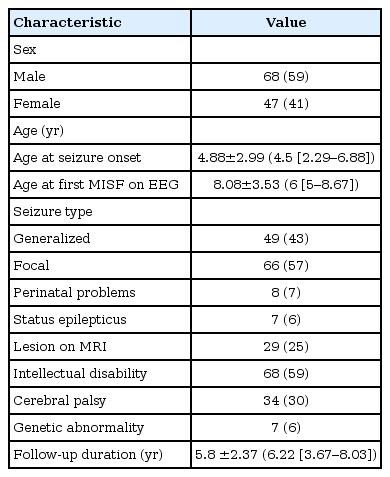
A total of 394 of patients were identified with MISF on EEG between November 1, 2008 and July 31, 2016. Among them, 115 who were diagnosed with epilepsy, treated, and followed up for more than 1 year, and did not meet the exclusion criteria were included in the current study. The patients' demographic profiles are shown in Table 1. The study population included 68 male patients and 47 female patients (ratio, 1.45:1). The mean onset age was 4.88±2.99 years, while the median age was 4.5 years (IQR, 2.29 to 6.88). The mean age at MISF on EEG was 8.08±3.53 years, while the median age at MISF on EEG was 6 years (IQR, 5 to 8.67). Seizure type was generalized in 49 (43%) patients and focal in 66 (57%). Of them, 29 patients (25%) had symptomatic epilepsy with lesions on brain MRI. Eight patients (7%) had a history of perinatal problems, 68 (59%) had intellectual disability, 34 (30%) had cerebral palsy, and seven (6%) had genetic disorders (Table 1).
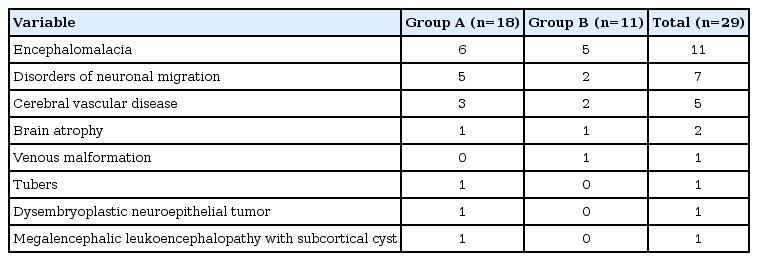
Lesion types revealed through MRI of the brain included periventricular leukomalacia in 11 patients, disorders of neuronal migration (cortical dysplasia, lissencephaly, polymicrogyria, pachygyria, heterotopia) in seven, cerebral vascular disease in five (including one patient with encephalomalacia suspected due to cerebral vascular disease), brain atrophy in two, and others (venous malformation, tubers, dysembryoblastic neuroepithelial tumor, and megalencephalic leukoencephalopathy with subcortical cyst) (Table 2).
The genetic disorders included tuberous sclerosis complex, Prader-Willi syndrome, deletions (deletion of 15q11.2-13.1, 45; XY, del(13, 14)(q10:q10)), 9p trisomy), and Van der Knaap syndrome (megalencephalic leukoencephalopathy with subcortical cyst).
2. Analysis of risk factors that influence therapeutic result
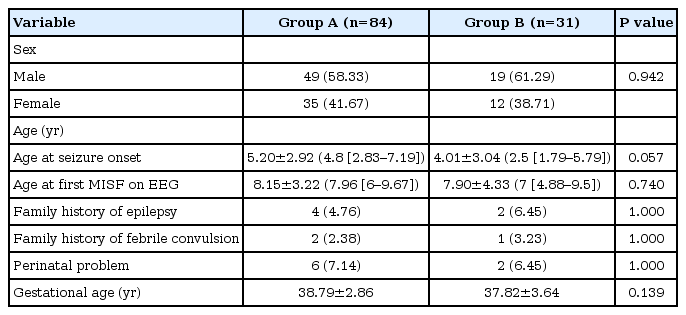
Among the total 115 patients, 84 (73%) were in group A and 31 (27%) were in group B. There was no intergroup difference in sex distribution; both groups showed a male predominance. The mean age at seizure onset was 5.20±2.92 years (median age, 4.88 [IQR, 2.83 to 7.19]) in group A versus 4.01±3.04 years (median age, 2.50 [IQR, 1.79 to 5.79]) in group B, showing no statistical significance (P=0.057). The mean age at which MISF was first detected on EEG was 8.15±3.22 years (median age, 7.96 [IQR, 6 to 9.67]) in group A versus 7.90±4.33 years (median age, 7 [IQR, 4.88 to 9.5]) in group B, showing no statistical significance (P=0.773). The clinical factors of family history of epilepsy and febrile convulsion, history of perinatal problems, and gestational age did not differ between groups (Table 3).
A higher proportion of patients in group B had generalized seizures (n=19 [61%]; P=0.025). The baseline frequency of seizures at the time of MISF detection on EEG was 4.02±17.52 times/month (median, 0 times/month [IQR, 0 to 0.33]) in group A versus 112.23±357.78 times/month (median, 1 [IQR, 0 to 45]) in group B (P=0.103). The duration from the first seizure to the start of AED did not differ between the groups (P=0.656) (Table 4).
Intellectual disability and cerebral palsy, which were suggested related to symptomatic or cryptogenic epilepsy, were more common in group B (P<0.001 and P=0.046, respectively). The presence or absence of lesions on MRI did not differ between groups (P=0.194). Six patients in group A versus one patient in group B had a genetic disorder (no statistically significant difference) (Table 5).
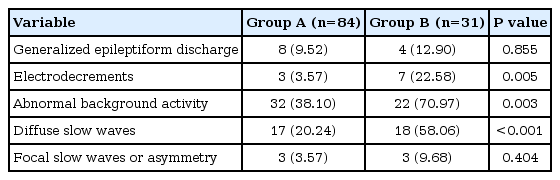
In EEG findings, electrodecrements and abnormal background activities such as diffuse slow waves and poorly organized sleep features were more common in group B (P=0.005 and P=0.003, respectively) (Table 6).
3. Clinical course and change in EEG findings with AED therapy
Total follow-up duration of overall patients was 5.8±2.37 years (median, 6.22 [IQR, 3.67 to 8.03]) (Table 7). It was 6.29±2.22 years (median, 7.21 [IQR, 4.89 to 8.17]) in group A versus 4.48±2.30 years (median, 4.07 [IQR, 2.62 to 6.14]) (Table 7). In group A, they had seizure-free period of 3.87±2.47 years (median, 3.14 [IQR, 2.06 to 5.71]) by last follow-up visit.
A higher number of patients received polytherapy of AED in group A (n=32 [38.10%]) than in group B (n=24 [77.42%]) (P=0.001). The mean number of AEDs used was 1.52±0.91 in group A versus 3.42±1.98 in group B (P<0.001) (Table 7).
The number of patients treated with anti-seizure therapy other than AED therapy such as a ketogenic diet or vagus nerve stimulation (VNS) was higher in group B than group A (P<0.001 and P=0.018) (Table 7) .
The findings of follow-up EEG showed improvements in MISF along with improvements in clinical seizure. Among total 109 patients who had follow-up EEG, 81 were in group A and 28 were in group B. More patients in group A (n=58 [71.6%]) than group B (n=10 [35.7%]) showed the disappearance of MISF on EEG. Furthermore, EEG findings were normal in more patients in group A (n=21 [25.9%]) than in group B (n=0 [0%]) (P=0.007) (Table 8).
Discussion
Very few studies have described the characteristics of patients with MISF since the study of Blume et al. [1,4] in 1970s. The current study aimed to elucidate the clinical characteristics and prognostic factors of patients with post-infantile epilepsy with good prognosis despite the presence of MISF on EEG. For this purpose, this study excluded patients with a history of infantile spasms, a relatively well-known epileptic syndrome with remarkable characteristics and poor prognosis. As a result, we included a considerable number of patients with MISF on EEG who had post-infantile epilepsy and a good response to AED therapy showing improvement in clinical seizures as high as two-thirds (group A, n=84 [73%]). Patients in the seizure control group required fewer AEDs (1.52±0.91) and were less likely to require adjuvant therapies such as a ketogenic diet and VNS.
In our study, the prognostic factors associated with a poor prognosis and poor seizure control were generalized seizures, premorbid intellectual disability or cerebral palsy, and EEG abnormalities such as electrodecrements and abnormal background activity.
Blume et al. [4] reported that half of their studied patients with MISF had daily and frequent seizures, while one-third had moderate to severe intellectual disability and were difficult to educate. They concluded that these were descriptive characteristics of patients with MISF, while the current study revealed that frequent seizures and intellectual disability were features highly suggestive of a poor prognosis but not characteristics of MISF specifically.
In the literature, the usefulness of EEG is well documented. For example, the EEG abnormality supports the prediction of seizure recurrence in the first unprovoked seizure and the diagnosis of epilepsy [7-9]. In addition to it, our study suggests two aspect of usefulness of EEG. First, abnormal findings additional to MISF are correlated with prognosis. Background abnormalities such as diffuse slow waves or abnormal sleep features were highly suggestive of poor prognosis in seizure control. Second, the improvement on EEG reflected the clinical progression of improvement. Our study showed that normalization or improvement of MISF on follow-up EEG was highly correlated with an improving clinical course. Two-thirds (72.6%) of the seizure control group was accompanied by improvement in MISF on EEG and one-fourth (25.9%) of the seizure control group had fully normalized EEG findings with AED therapy. We suggest that EEG is useful for predicting prognosis of seizure control and estimating clinical improvement with AED therapy.
A recent analysis of MRI lesions and EEG findings in epilepsy patients described that patients with multifocal interictal epileptiform discharges on EEG had a statistically significantly higher rate (68%) of abnormalities on MRI [10]. This was somewhat different from our result that 25% of patients have a lesion on MRI, similar to the 21% to 31% of the overall pediatric patients who visited with a first unprovoked seizure [11-14]. In cases of pediatric epilepsy, the rate of abnormalities on brain MRI increased [10]. The reasons that our study findings suggested that the presence of a lesion on MRI was not significantly associated with seizure control might be due to patients with significantly severe lesions being excluded by exclusion criteria of whom diagnosed infantile spasms and early-onset seizures before 1 year of age; thus, a relatively small number of patients with less severe abnormalities on brain MRI were included in our study.
Blume et al. [1] reported that a considerable number of patients with findings of MISF on EEG had generalized seizures. Several strudies described that the major seizure type of SE-MISF was brief generalized tonic seizure [5,15-17]. Conversely, in the current study, the percentage of patients with focal seizures was higher than previously reported, while the focal seizure type was a proven good prognostic factor for seizure control. This suggests that seizure type is a major clinical characteristic that influences prognosis.
Since 1992, several studies suggested that epileptic patients with clinical characteristics, especially a severe clinical course and MISF on EEG could be defined as an epileptic syndrome [5,15-18], primarily LGS or SE-MISF, which differs in several ways. In 2006, Yamatogi and Ohtahara [6] defined SE-MISF and summarized its characteristics [6]. SE-MISF is defined as symptomatic generalized epilepsy with age-specific epileptic encephalopathy. They described the characteristics of SE-MISF as: (1) MISF, diffuse slow background activity, and rare diffuse epileptic discharges on EEG; (2) frequent generalized minor seizures as the main seizure type; (3) mutual transition between the age-dependent epileptic encephalopathies (i.e., Ohtahara syndrome, West syndrome, and LGS); (4) early onset of epilepsy (despite variable SE-MISF onset); (5) association with intellectual disability and neurological abnormality; (6) variable and non-specific etiology; and (7) intractable seizure and psychomotor deterioration.
Likewise, the current study showed that the characteristics associated with a poor prognosis were abnormal background activity on EEG, generalized seizures, and intellectual disability. These are similar to the characteristics of SE-MISF and accounted for three of seven characteristics mentioned by Yamatogi and Ohtahara [6].
Despite SE-MISF not being widely accepted as an epileptic syndrome, efforts to categorize patients with MISF as epileptic syndromes are increasing with our understanding of them. Similarly, our study emphasized that MISF on EEG is not strongly correlated with LGS, SE-MISF, or intractable seizures. Thus, we conclude that MISF should be interpreted correctly and precisely according to individual clinical characteristics, which would prevent errors in the estimation of prognosis during diagnosis and the early phase of treatment.
In current study, seizure control was defined as seizure freedom over 6 months by the last visit. This definition might reflect a short-term outcome in some patients, but in fact the subject had various follow-up periods and seizure-free periods because this study was retrospective. If a prospective study with a long-term follow-up period is conducted, we can further understand the characteristics of benign and severe epileptic patients with MISF and more concise statistics related to the prognosis, such as seizure recurrence, progression of seizure frequency of epileptic encephalopathy, possibility of AED cessation, a long-term seizure-free period, and finally epilepsy cure.
A further study is required to elucidate the clinical significance of MISF in patients with good prognosis, particularly the pathogenesis of epilepsy in cases of idiopathic generalized epilepsy or focal onset seizures.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JK and SON. Data curation: JK, YJL, AK, YMK, and GMY. Formal analysis: JK and YJL. Methodology: JK and SON. Project administration: JK, YJL, AK, GMY, and SON. Visualization: JK. Writing-original draft: JK. Writing-review & editing: JK and SON.
Acknowledgements
This work was supported by a 2-Year Research Grant of Pusan National University.