Short Course and Early Switch of Vigabatrin for Infantile spasms
Article information
Abstract
Purpose
Vigabatrin has proven efficacy in the treatment of infantile spasms, but it carries the risk of irreversible visual field constriction. The incidence of vigabatrin-induced visual field constriction seems to depend on the extent of vigabatrin exposure. The aim of this study was to evaluate whether the therapeutic effect of vigabatrin is maintained in patients with infantile spasms receiving a short vigabatrin course followed by switching to another antiepileptic drug.
Methods
Patients with infantile spasms responsive to initial vigabatrin treatment were divided into a vigabatrin switch group (n=25) and a vigabatrin maintenance group (n=41). In the former group, vigabatrin was switched to other drugs within 6 months of spasm remission. The rate of seizure recurrence at 6 and 12 months from spasm remission was compared between the two groups.
Results
No statistically significant differences were found between the vigabatrin switch and maintenance groups in the age of onset, presence of concomitant seizures, time from spasm onset to vigabatrin treatment, time from vigabatrin treatment initiation to spasm remission, or vigabatrin dose at spasm remission. The number of patients with seizure recurrence at 12 months after spasm remission was 3 (3/25, 12%) in the vigabatrin switch group and 10 (10/41, 24.4%) in the vigabatrin maintenance group. The seizure recurrence rate at 12 months from spasm remission was not significantly different between groups.
Conclusion
A short course of vigabatrin could be considered in patients with infantile spasms who are responsive to initial vigabatrin treatment, since spasm remission was maintained after switching to other drugs.
Introduction
Vigabatrin (VGB) has proven efficacy in the treatment of infantile spasms that is resistant to conventional antiepileptic drugs (AED) [1]. No difference in long-term outcome was found between treatment with VGB or with adrenocorticotropic hormone (ACTH) [2-4]. Considering the serious side effects of ACTH such as infection, hypertension, osteoporosis, or electrolyte disturbances, VGB is a good choice for the treatment of infantile spasms [5,6].
However, VGB has risk of visual field constriction (VFC) as an adverse effect. Of the patients treated with VGB, 44% (738/1,678) had VFC [7]. VGB-induced visual field defect is irreversible, and its incidence increases with the duration and total dose of VGB [8,9]. In a study examining the visual fields in school age children who had received VGB in infancy, visual field defects were found in 9% of children who received VGB for 1 year or less, in 30% of children who received VGB for 12 to 24 months, and in 63% of children who received VGB for more than 2 years [10]. In an observational cohort study using electroretinogram (ERG) to assess the VGB-induced retinal toxicity, 5.3% and 13.3% of children developed retinal damage with 6 and 12 months of VGB treatment [11].
Patients receiving VGB treatment require regular visual field examination; however, perimetry is impossible in young children or in children with cognitive impairment. ERG, which can monitor the retinal toxicity in young children, is difficult to perform because it requires general anesthesia [12].
Since the incidence of VFC depends on the duration of VGB treatment, if we use a short-term VGB treatment paradigm, it will be possible to reduce the risk of VFC. Controlled studies on the optimal duration of VGB to maintain the therapeutic effect without adverse effect were not found. In a long-term (mean 5.25 years) follow-up of 21 patients with infantile spasms, three patients had relapse of spasms, all within the first 6 months [13]. In another study, VGB was stopped after 6 months without relapse in patients with infantile spasms with Down syndrome, cryptogenic etiology, or neonatal hypoxic-ischemic encephalopathy [14,15].
Although VGB is effective for the treatment of infantile spasms, the risk of irreversible adverse events increases with the duration of treatment. Hence, if the recurrence rate does not increase in patients with a short treatment course of VGB followed by switching to another AED when compared with patients with a long-term VGB treatment, it could be a new strategy for treating infantile spasms. The purpose of this study was to evaluate whether the recurrence rate increases after switching to another AED from VGB compared to long-term VGB treatment in patients with infantile spasms.
Materials and Methods
This study was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. H-1503-033-654). Written informed consent by the patients was waived due to a retrospective nature of our study.
Medical records of patients newly diagnosed with infantile spasms and treated with VGB in the Seoul National University Hospital from January 1997 through May 2014 were reviewed retrospectively. Infantile spasms were defined as the follows: (1) epileptic spasm onset during infancy at less than 12 months of age; (2) spasms confirmed either by history or by video electroencephalography (EEG) monitoring; and (3) hypsarrhythmic pattern on interictal EEG. Patient inclusion criteria included being a VGB responder and having a follow-up period of at least 12 months from spasm free. VGB responder was defined as (1) spasm free within 1 months of starting on VGB; (2) remaining spasm free for more than 1 months; and (3) resolution of hypsarrhythmic pattern on EEG. For the patients taking other AED before VGB start, patients without change of dose during were included. Patients with other additional AEDs after the start of VGB were excluded. Also, patients treated with corticosteroids were not included.
Gender, date of birth, age at spasms onset, etiology, EEG record, time from spasms onset to VGB treatment initiation, time from VGB treatment initiation to spasm free, VGB dose at spasm free, and relapse at 6 months and 12 months from spasm free were reviewed.
Patients were divided into two groups: VGB early switch group (switch group) and VGB maintenance group (maintenance group). VGB was switched with other drugs within 6 months of spasms remission in the VGB early switch group. The recurrence rate of spasms and other seizures at 6 and 12 months from spasms remission was compared between the two groups. In addition, risk factors such as gender, VGB switch, age of onset, etiology, and concomitant seizures were compared between patients who relapsed at 12 months and those who did not.
Statistical analysis was performed using SPSS version 22.0 for Windows (IBM Co., Armonk, NY, USA). A t-test for comparison of means and chi-square test or Fisher’s exact test for comparison of proportion between groups were used. Statistical significance was defined as P<0.05.
Results
1. Baseline characteristics
Two hundred and three children were newly diagnosed with infantile spasms and treated with VGB between January 1997 and May 2014. Seventy patients were VGB responder, and 66 patients satisfied inclusion criteria. Two patients who had less than 12 months follow-up and two patients for whom VGB was switched with other drugs between 6 and 12 months of spasms remission were excluded.
Of the 66 patients included in the analysis, 25 patients (12 boys, 13 girls) were in the switch group, and 41 patients (24 boys, 17 girls) were in the maintenance group. The mean age of infantile spasms onset was 6.1 months in the switch group, and 5.8 months in the maintenance group (P=0.562). The mean time between onset of spasms and initiation of VGB was 24.5 days in the switch group, and 35.2 days in the maintenance group (P=0.243). The mean time between initiation of VGB and spasm free was 11.9 days in the switch group, and 13.5 days in the maintenance group (P=0.476). The mean VGB dose at spasm free was 70.5 mg/kg/day in the switch group, and 64.9 mg/kg/day in the maintenance group (P=0.451). Six patients in each group had concomitant seizures when spasms occur (P=0.348).
The number of symptomatic cases was 12 (tuberous sclerosis complex [TSC] two, congenital malformation one, brain injury eight, chromosome abnormality [Down syndrome] one) in the switch group, and 27 (TSC nine, congenital malformation seven, brain injury eight, central nervous system infection three) in the maintenance group.
The mean duration between spasm free and tapering VGB was 2.5 months (range, 0.5 to 5.0), and VGB was stopped after an average of 4.5 months (range, 1.4 to 7.5) from spasm free. In the switch group, VGB was replaced with zonisamide (n=13), levetiracetam (n=11), and topiramate (n=1) (Table 1).
2. Outcome
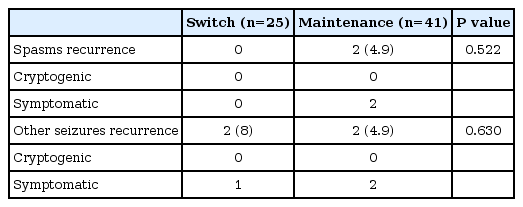
At 6 months from spasm free, the number of patients with recurrence of spasm was two, and the number of patients with recurrence of other seizure was four (Table 2). At 12 months from spasm free, the number of patients with recurrence of spasm was six, and the number of patients with recurrence of other seizure was seven (Table 3). The average period from spasm free to recurrence was 8.4 months (range, 4.6 to 12.0), and the average period to other seizure recurrence was 6.0 months (range, 2.8 to 9.0).
3. Risk factors for seizure recurrence
After 12 months from spasms remission, 13 of 66 patients developed subsequent seizures: six patients had spasms and seven patients had other types of seizures. Clinical characteristics of patients by recurrence are shown in Table 4. There was no statistically significant difference between the recurrence group and the remission group. The number of patients in the switch group was three (23%) with recurrence and 22 (42%) without recurrence (P=0.340). The number of symptomatic cases was 10 (77%) with recurrence and 29 (55%) without recurrence (P=0.144). When patients who experienced recurrence at 12 months after spasms had controlled were compared to patients without recurrence, no statistically significant difference was found, but recurrence tends to occur more frequently in cases where the cause of spasms was symptomatic.
Discussion
VGB is effective in infantile spasms but can cause irreversible retinal toxicity manifested by VFC. Moreover, previous studies have reported that longer duration of medication increases the risk of retinal toxicity [8-10]. Recent research has shown that there is a difference in the frequency of visual field defect at 6 months after VGB medication and that after 12 months [11]. This indicates that vision loss can occur even if treatment has not been prolonged for years. However, there is a lack of research on the duration of medication to maintain therapeutic effects without increasing the risk of an adverse effect. In a study, VGB was stopped after 3 to 6 months in 19 patients who were responding to VGB. There was no case of recurrent spasms, although follow-up duration was not long, 13 to 50 months [14]. However, in a study on long-term prognosis, there was more than 50% recurrence in the form of epilepsy, including partial seizures [3,16]. So, it is often required treatment for subsequent seizure after the spasm remission. For infantile spasms patients who were successfully treated with VGB, short term VGB with followed another AED may be considered. If the recurrence rate does not increase after switching to another AED compared to long-term VGB treatment, it could be a new treatment strategy that reduces the risk of VFC and maintains the therapeutic effect.
We changed the medication to a different anticonvulsant drug in infantile spasms patients who were successfully treated with VGB within 6 months, and compared these patients to those who continued VGB, for any difference in the recurrence rate of spasms and other seizures. Although there is no guideline for the optimal treatment duration of VGB, limited studies suggest VGB could be stopped after 6 months without a relapse [14,15] and incidence of VFC seems to increase after 6 months of VGB treatment [11]. There was no statistically significant difference between the two groups with respect to baseline characteristics and recurrence rate at 6 and 12 months after spasms controlled.
Although no medications have been proven to be effective for infantile spasms except for ACTH and VGB, there are cases where infantile spasms were treated with medications that are effective for partial seizures, such as topiramate, zonisamide, and levetiracetam [17-22]. More than 50% of the patients with a history of infantile spasms develop chronic epilepsy, most of them have other types of seizures than spasms. AED that is effective for concomitant with spasms or other types of seizures that appear after infantile spasms is controlled should be considered as switch drug.
This study is a retrospective study, and has limitations in that the duration of VGB treatment and the switch in drug were not constant in the switch group and the follow-up period was short. However, our data suggest that short course of VGB followed by switching to another AED can be a new strategy for treating infantile spasms. In future research, long-term prognosis and the difference in frequency of VFC between the two groups should be assessed.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: HWR, BCL, and KJK. Data curation: HWR and BCL. Formal analysis: HWR and BCL. Methodology: HWR, BCL, and KJK. Project administration: HWR, BCL, and KJK. Visualization: HWR, BCL, and KJK. Writing - original draft: HWR and KJK. Writing - review & editing: HWR, HK, BCL, HH, JHC, JEC, and KJK.