Reconsideration of Vigabatrin Effect in Infantile Spasms Treatment
Article information
Abstract
Purpose
To investigate the effect of vigabatrin (VGB) as a therapeutic agent for patients with infantile spasms (IS), compare risk factors for treatment response, and review safety of VGB by assessing its side effects.
Methods
Among 35 patients admitted to the Department of Pediatric Neurology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea who received initial monotherapy with VGB under diagnosis of IS, 23 patients who met our inclusion criteria were enrolled and their medical records were retrospectively reviewed.
Results
Of these 23 patients, average age at diagnosis was 7.26±4.8 months and average age at spasms was 6.20±3.8 months. Average treatment lag was 1.09±1.8 months. Thirteen patients (56.5%) achieved seizure free status. There was no ophthalmic complication among patients. Remission of hypsarrhythmia at 3 and 6 months after treatment was a good prognostic factor (P=0.026 and P=0.004, respectively).
Conclusion
VGB is effective enough to become a first-line drug for children with IS. Better prognosis can be expected in patients with clinical remission of hypsarrhythmia on electroencephalography after treatment initiation using VGB compared to those who do not have such remission. Regular eye examination and follow-up check-up are also needed in parallel with the use of VGB.
Introduction
Infantile spasms (IS) are intractable epilepsies classified as epileptic encephalopathy in the International League Against Epilepsy (ILAE) [1]. They typically present three clinical features: epileptic spasms, developmental delay, and the presence of hypsarrhythmia on electroencephalography (EEG) under 2 years old [2]. They do not respond well to conventional anticonvulsant. Thus, adrenocorticotrophic hormone (ACTH), steroid, and vigabatrin (VGB) have been used as initial therapeutic agents [3].
VGB was first marketed for treating intractable complex partial seizure and IS in the United Kingdom (UK) in 1989. VGB is an irreversible, selective inhibitor of enzyme-activated gamma aminobutyric acid transaminase. It suppresses gamma aminobutyric acid transaminase catabolism and enhances the activation of interneurons by increasing availability of gamma aminobutyric acid in synaptic cleft [4]. In addition, VGB can partially inhibit mammalian target of rapamycin and glial proliferation in animal model of tuberous sclerosis complex [5]. However, some studies have shown increased risk of peripheral visual field defects (VFDs) by VGB in adults and children [6]. Thus, the use of VGB has been limited for a while. The frequency of VFDs has been reported to be 21% to 34% in Western studies [7-9]. Taurine deficiency [10,11] and reversible magnetic resonance imaging (MRI) changes [12,13] might play considerable roles in the development of VFDs. Nevertheless, it is inadequate to conclude that all ethnicities in the world will develop VFDs after using VGB as there have been no collected baseline data of patients prior to VGB administration. In 2009, the United States Food and Drug Administration approved the use of VGB as a monotherapy in 1 month old to 2 years old children with IS since VFDs seemed to be related to prolonged administration of VGB and accumulated quantity of VGB in the body [14]. In 2013, VGB was also approved as a therapeutic agent for older patients (>10 years old) with intractable complex partial seizures.
The use of ACTH for patients with IS is impossible in South Korea at present time. Therefore, there are very limited options for patients with IS in our country. Recently, it has been reported that the incidence of VFDs is not significantly increased when VGB is used for a short period of time [15-17]. Therefore, we need to reconsider the use of VGB as a therapeutic agent for patients with intractable epilepsy including IS. Thus, the objective of this study was to investigate the effect of VGB in patients with IS and identify factors closely related to the treatment by comparing two groups (treatment responder group and treatment non-responder group). In addition, the safety of VGB was reviewed by assessing its side effects.
Materials and Methods
1. Study subjects
Among 35 patients admitted to the Department of Pediatric Neurology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea from April 2009 to June 2018 who received initial monotherapy with VGB under the diagnosis of IS, 23 patients (13 males, 10 females) aged 2 months old to 2 years old who had follow-up check-up for 6 months or more were enrolled. Exclusion criteria were: (1) any symptom or EEG interpretation inappropriate for diagnosis of IS, (2) initial therapy using agents other than VGB, and (3) patients who failed to make follow-up check-up for 6 months or more.
All participants were referred to the Department of Ophthalmology for eye examination prior to treatment and at 3 and 6 months after initiation of treatment. There was a limitation for visual field examination due to patient’s age. Thus, visual adverse effects were identified through visual evoked potential (VEP), funduscopic examination, and guardian’s questionnaire.
This was a retrospective study with data collected from medical records of participants. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea (IRB number: KC17RESI0535). Written informed consent by the patients was waived due to a retrospective nature of our study.
2. Methods
IS was diagnosed based on clinical features and EEG findings of patients. The feature of epileptic spasm is a brief, bilateral symmetrical contraction of muscles involving neck, body, and extremities. In addition, there must be a presence of hypsarrhythmia or modified hypsarrhythmia on EEG. Based on onset age of seizures, participants were divided into four groups: 6, 6 to 12, 12 to 18, and 18 to 24 months.
Potential risk factors such as age of onset, age at diagnosis, sex, birth history, presence of developmental delay, brain MRI findings, treatment lag (delayed period between clinical onset of spasms and initiation of treatment), presence of hypsarrhythmia on EEG, and clinical remission of hypsarrhythmia after the treatment were used to examine their associations with VGB treatment response. Participants were divided into two groups according to outcome of treatment. Each potential risk factor was statistically analyzed by comparing the two groups. All patients underwent Bayley scales of Infant Development-II test to assess developmental delay. Patients who showed 25% or more delay in one developmental area were defined as having developmental delays.
Initial dose of VGB was 40 to 50 mg/kg/day administered in two divided doses. The dose of VGB was gradually increased every 2 days to a target dose of 100 mg/kg/day. However, if the patient responded to a given lower dose of medication, the dose was maintained without further increase. When a patient did not respond to a dose of 100 mg/kg/day, the patient was treated with polytherapy in combination with other treatments such as steroid pulse therapy without increasing VGB dose.
The effect of VGB treatment on participants were evaluated at 6 months after the treatment based on decrease in frequency of spasms as a clinical evaluation criterion. Participants were divided into three groups based on the decrease in frequency of spasms: group 1, completely resolved; group 2, resolved more than 50%; and group 3, resolved less than 50%. For statistical analysis, patients in groups 2 and 3 were assigned to treatment non-responder group. IBM SPSS version 24.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. To distinguish the difference between treatment responder group and treatment non-responder group, independent t-test was used when normality assumption was available whereas Mann-Whitney U test was used when the normality assumption was unavailable. Chi-square test was used to determine the relationship with independent variables. Result was considered statistically significant when P value was less than 0.05.
Results
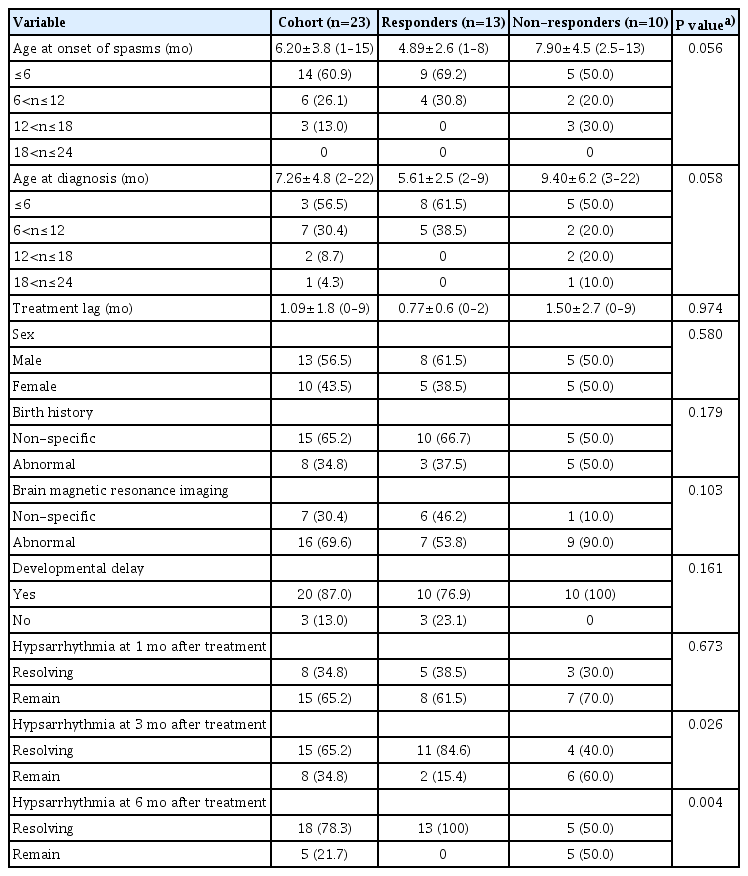
From April 2009 to June 2018, 35 patients who were admitted to the Department of Pediatric Neurology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, received initial monotherapy with VGB under the diagnosis of IS. Only 23 patients (males 13, females 10) met the study criteria. Data of each patient including sex, birth history, developmental delay, brain MRI findings are shown in Table 1.
Of these 23 patients, their average age at spasms was 6.20±3.8 months. Among age groups, those who were less than 6 months old accounted for the most (60.9%, 14 patients). Average age at diagnosis of IS was 7.26±4.8 months. Twenty patients (86.9%) were diagnosed with IS before 12 months of age and 13 patients were diagnosed before the age of 6 months old. Treatment lag time ranged from less than 1 to 9 months, with average treatment lag of 1.09±1.8 months.
Birth complications were reported in eight patients (34.8%), including five cases (62.5%) of prematurity, four cases (50%) of hypoxic-ischemic encephalopathy, three cases (12.5%) of intraventricular hemorrhage, and one case (12.5%) of subgaleal hemorrhage. In addition, 20 of 23 patients (87.0%) had developmental delay. Abnormal brain MRI findings were shown in 16 patients (69.6%). The most common abnormal findings were hypoxic ischemic encephalopathy (five cases) and periventricular leukomalacia (three cases).
Response of patients to VGB treatment was evaluated based on frequency of spasms. Number of patients in groups 1 (completely resolved), 2 (resolved more than 50%), and 3 (resolved less than 50%) were 13 (56.5%), six (26.1%), and four (17.4%), respectively. There was no difference in dose of VGB between the groups. No patient showed significant side effects to discontinue the drug. All patients had normal ophthalmologic findings.
Clinical remission of hypsarrhythmia on EEG was also checked. One month after using VGB, the pattern of hypsarrhythmia was resolved in eight patients (34.8%). After 3 months of treatment with VGB, 15 patients (65.2%) showed clinical remission of hypsarrhythmia. After 6 months of VGB treatment, five of 23 patients (21.7%) still had hypsarrhythmia.
Analysis of age distribution at diagnosis showed that the average age at diagnosis in the treatment responder group (5.61±2.5 months) was younger than that in the treatment non-responder group (9.40±6.2 months). Onset age of seizures was also younger in the treatment responder group (4.89±2.6 months) than that in treatment non-responder group (7.90±4.5 months). All patients in the treatment responder group started treatment for seizure before 12 months of age. However, neither age at diagnosis nor onset age showed statistically significant association with treatment response (P=0.058 and P=0.056).
Average treatment lag was 0.77±0.6 months in the treatment responder group (13 patients) and 1.50±2.7 months in the treatment non-responder group (10 patients). Although treatment lag was longer in the treatment non-responder group than that in the treatment responder group, the difference between the two was not statistically significant (P=0.056).
In the treatment responder group (13 patients), three patients (37.5%) presented with abnormal birth history. In the treatment non-responder group (10 patients), five patients (50%) had remarkable birth history. Although birth history was more remarkable in the treatment non-responder group, the different was not statistically significant (P=0.179). Seven patients (53.9%) in the treatment responder group and nine patients (90.0%) in the treatment non-responder group had abnormal brain MRI findings. Ten of 13 patients (76.9%) in the treatment responder groups and 10 patients (100%) in the treatment non-responder group had developmental delay. However, neither brain MRI findings nor developmental delay showed statistically significant differences between the two groups (P=0.103 and P=0.161, respectively).
Regarding clinical remission of hypsarrhythmia, at 1 month after treatment, 61.5% (eight patients) in the treatment response group and 70% (seven patients) in the treatment non-responder group still presented with hypsarrhythmia, showing no significant difference between the two groups (P=0.673). At 3 months after treatment, 84,6% (11 patients) in the treatment responder group appeared to have clinical remission of hypsarrhythmia whereas 40% (four patients) in the treatment non-responder group presented clinical remission of hypsarrhythmia on EEG, showing statistically significant difference between the two groups (P=0.026). EEG test performed at 6 months after the initiation of treatment revealed that all patients (100%) in the treatment responder group and 50% patients in the treatment non-responder group had clinical remission on hypsarrhythmia, showing statistically significant difference between the two groups (P=0.004).
Discussion
ACTH, steroid, and VGB are considered as drugs of choice for IS. However, ACTH is currently unavailable in South Korea while high dose of steroid is known to cause various side effects on endocrine system of patients. On the other hand, VGB is an initial therapy that is effective and relatively safe in children with IS. Previous studies have reported that about 35% to 80% of patients become seizure free after treating with VGB [18-20]. In our study, 56.5% of patients achieved seizure free when they used VGB as initial monotherapy, showing effects comparable to those of past researches.
There are various potential risk factors for IS, including sex, history of neonatal seizures, age of onset, abnormal brain MRI findings, treatment lag, and accessibility to medical institution for treatment [18,21,22]. The present study revealed that patients with clinical remission of hypsarrhythmia after 3 months from the initiation of treatment with VGB had better treatment response overall, supporting many articles showing that hypsarrhythmia was an important prognostic factor [22-25]. At 1 month after initiation of treatment with VGB, hypsarrhythmia was only resolved in 34.8% of patients. There was no statistically significant treatment response. However, EEG test performed at 3 months after the initiation of treatment with VGB revealed that 65% to 79% of patients showed clinical remission of hypsarrhythmia and achieved seizure free, consistent with existing researches. Therefore, regular evaluation is important to determine patient’s outcome even if hypsarrhythmia persists in EEG test.
Many animal studies and retrospective studies have discussed bilateral VFDs due to use of VGB [6,10-14]. However, no patient in the present study experienced such adverse drug reaction when regular eye examination was performed, consistent with a small prospective study recently conducted in Japan [16]. In addition, a recent study has reported that VFDs are cause by the disease itself rather than the use of VGB [17]. However, that study had limitations such as small sample size, short duration of study period, and lack of patient’s cooperation for eye examination that might have resulted in inaccuracy of eye examination. Hence, different methods such as VEP, funduscopic examination, and electroretinography need to be used to compensate the limitation of eye examination on children. Regular follow-up check-up is necessary even after completing treatment with VGB.
In conclusion, VGB is effective enough to become a first-line drug for children with IS. Better prognosis can be expected for patients with clinical remission of hypsarrhythmia on EEG after treatment initiation using VGB. Regular eye examination and follow-up check-up are also needed in parallel with the use of VGB. Since this is a retrospective study by analyzing medical records of a small number of patients, large-scale studies are needed in the future.
Notes
No potential conflicts of interest relevant to this article was reported.