Brain Magnetic Resonance Imaging in Indonesian Patients with Cerebral Palsy
Article information
Abstract
Purpose
Cerebral palsy (CP) is a neurological health problem that affects children around the world and warrants particular attention. Magnetic resonance imaging (MRI) can be used to visualize brain disorders, as it provides a clear view of the brain’s anatomy, including the location of any damage or structural abnormalities. The objective of this study was to obtain a detailed MRI representation of patients with CP.
Methods
This study employed a retrospective design involving the examination of medical records. It included CP patients aged between 1 and 16 years who had undergone brain MRI examinations. The Gross Motor Function Classification System scale was used to classify limitations in functional motor ability among these patients. The brain MRI results were categorized as either normal or abnormal.
Results
Of 60 CP cases, 50% were classified as severe, with the remaining cases considered mild to moderate. Epilepsy was present in 66.7% of patients with severe CP. The most common type of CP, among both mild-to-moderate and severe cases, was quadriplegia. Gray matter lesions on brain MRI were more common in severe than mild-to-moderate cases, while vascular insult lesions and brain malformations were less frequent. A significant difference was observed in the severity of CP when a gray matter lesion was present on MRI.
Conclusion
Most children with CP exhibited abnormal results on brain MRI. Lesions of the white and gray matter were the most frequently observed. MRI plays a crucial role in understanding the underlying pathological brain abnormalities in CP.
Introduction
Cerebral palsy (CP) is a neurological health problem that affects children around the world and that warrants particular attention. It is characterized by permanent neurological impairments that impact movement and posture, leading to non-progressive physical limitations during a child’s brain development [1,2]. The clinical manifestations of CP may include disturbances in sensation and cognition, in addition to motor disability. The causes of CP are typically associated with brain injury during the prenatal, perinatal, and postnatal periods. The prevalence of this condition is estimated to be between 1.5 and 3 per 1,000 live births [2,3]. A study conducted in Islamabad, Pakistan reported 89 CP cases over an 18-month research period [4]. In Indonesia, the prevalence of CP is unclear due to the absence of a national registry. However, available data from Surabaya, Indonesia indicated 48 CP cases over a 2-year research period [5-7]. CP can potentially lead to socioeconomic issues as well as mental health problems for affected children and their families. Therefore, efforts to reduce the number of affected children, minimize the severity of the condition, and improve the quality of life are necessary at both societal and individual levels.
CP is associated with neurological disorders of the brain [8-10]. Magnetic resonance imaging (MRI) of the head is particularly helpful in thoroughly characterizing brain disorders [11-13]. MRI can vividly depict the brain’s anatomy, pinpoint the location of brain damage, and identify the presence of cerebral edema or other structural abnormalities, such as periventricular leukomalacia (PVL) and hydrocephalus. As a non-invasive diagnostic tool, MRI can forecast neurological deficits, even in early infancy [13,14]. It is a valuable instrument for diagnosing the cause and progression of abnormal brain development in cases of antenatal, perinatal, and newborn neuronal damage. Despite being a costly and time-intensive procedure, the clarity of the data obtained from an MRI surpasses that of a computed tomography scan. MRI studies in various types of CP have revealed a range of abnormalities, including structural malformations and neuronal cortical/subcortical damage. MRI can offer substantial insights into structural abnormalities and patient outcomes in clinical practice. However, a dearth of data exists on MRI features of CP in developing countries, like Indonesia. Establishing these data would enhance our ability to manage CP and promote the development of neuroprotective agents targeted at specific cerebral regions and timeframes. The objective of this study was to acquire a detailed MRI profile of patients with CP.
Materials and Methods
1. Design
This study employed a retrospective design involving medical records. The research was conducted at the outpatient and inpatient facilities of Dr. Soetomo Hospital General Academic Hospital, located in Surabaya, Indonesia.
2. Patients
The study sample included patients with CP aged between 1 and 16 years who had undergone MRI examination of the head. CP refers to permanent neurological disorders that affect movement and posture, resulting in non-progressive physical limitations that occur during a child’s brain development. The Gross Motor Function Classification System (GMFCS) scale is used to classify functional motor limitations in CP cases. This scale ranges from grade 1 to grade 5. Patients are classified as GMFCS level 1 if they can walk without meaningful impediments; level 2 if they can walk with some limitations; level 3 if they require a hand-held mobility device; level 4 if they can move independently but with limited abilities (they may use powered mobility); and level 5 if they can move only with the assistance of a wheelchair. In the present study, a pediatric neurologist determined the diagnosis of CP and the GMFCS level. A GMFCS scale score of 4 or 5 indicates severe CP, while a score of 1 to 3 suggests mild to moderate CP. Nutritional status was determined based on weight-for-age and length-for-age measures according to the CP growth chart.
3. MRI classification
The device used for MRI, including magnetic resonance spectroscopy and perfusion, was the MR360 Optima 1.5T (GE Healthcare, Chicago, IL, USA). An expert neuroradiologist interpreted the brain MRIs. The results were classified as either normal or abnormal. The abnormal MRI category encompassed lesions in the gray matter, characterized by either a hyperintense/hypointense signal or a reduction in volume in the superficial gray matter, deeper gray matter, or both. It also included lesions in the white matter, identified by a hyperintense/hypointense signal or a decrease in volume in the periventricular or deeper white matter regions, or the presence of ventricular enlargement or cysts. Lesions suggestive of vascular injury were also classified as abnormal if they exhibited a hyperintense/hypointense signal or a reduction in volume in the affected area, in accordance with its vascularity. This category included lesions resulting from hemorrhage, ischemia, or sinus thrombosis. Brain malformations were considered abnormal if unusual structural formations were present in the brain. These include abnormalities in the gyrus, such as pachygyria, polymicrogyria, or lissencephaly; schizencephaly; abnormalities of the corpus callosum and cerebellum; and hydrocephalus.
4. Ethics
This research was registered with and received approval from the Ethical Committee of Medical Professional Research at Dr. Soetomo Hospital in Surabaya, Indonesia, under the reference number 211-Year 2017Panke.KKE-III. Written informed consent by the patients was waived due to a retrospective nature of our study.
5. Statistics
This study employed analytical descriptive statistics. We analyzed relationships using the chi-square test and the Kruskal-Wallis test in SPSS version 24 (IBM Corp., Armonk, NY, USA). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Results
Of 60 patients with CP, 50% were classified as having severe CP, while the remainder were categorized as mild to moderate cases. The majority of these patients, regardless of CP severity, were self-referred to the hospital and resided in Surabaya. The most common etiology underlying severe CP was found to be prenatal issues, such as congenital infections. Perinatal problems related to birth asphyxia and prematurity, as well as postnatal complications due to brain infections and seizures, were also identified. A history of seizures, as evidenced by an epilepsy diagnosis, was present in 66.7% of patients with severe CP. The most common type of CP, both in mild-to-moderate and severe cases, was quadriplegia (Table 1).
The interpretation of brain MRI results revealed a higher prevalence of gray matter lesions in severe cases of CP, while white matter lesions were more frequently observed in mild to moderate CP cases (Fig. 1). Additionally, vascular insult lesions and brain malformations were detected, albeit less frequently (Table 2). A significant relationship was noted between the severity of CP and the presence of a gray matter lesion on the MRI.
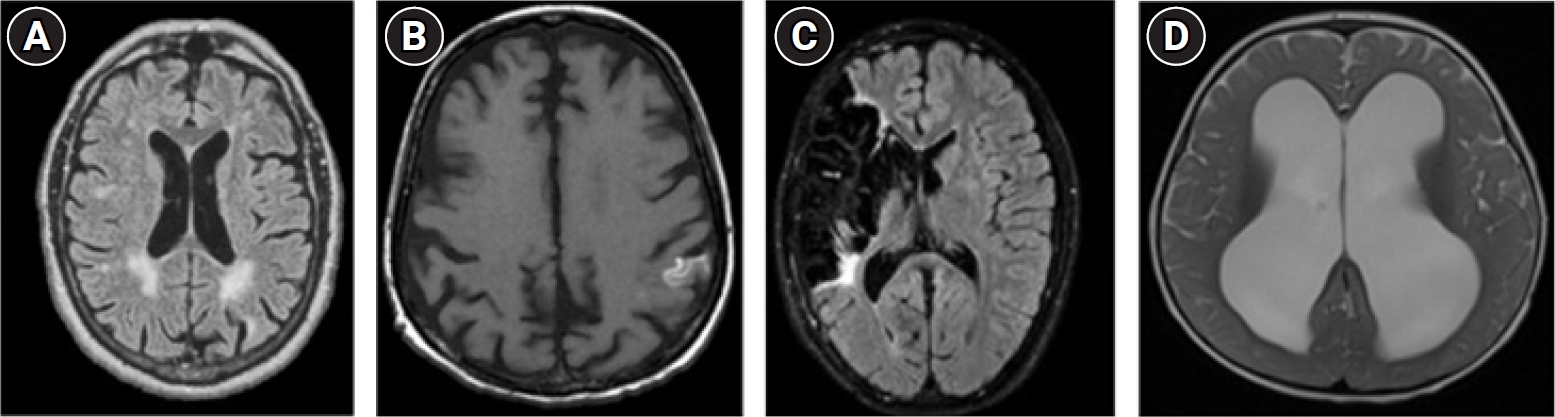
Magnetic resonance imaging findings in patients with cerebral palsy. (A) Periventricular white matter lesion. (B) Cortical hyperintensity categorized as a gray matter lesion. (C) Brain ischemia due to vascular insult. (D) Hydrocephalus categorized as a brain malformation.
Abnormal electroencephalogram findings were observed in 66.70% of patients with severe CP and 56.70% of patients with mild to moderate CP (Table 1). However, no significant correlation was observed between these results and the interpretation of brain MRI scans (P=0.198).
Discussion
MRI is reported to have a high sensitivity for the prediction of CP. In studies conducted by Robinson et al. [15] and Reid et al. [11], the frequencies of MRI abnormalities in CP were approximately 84% and 86%, respectively. However, in the present study, the proportion of MRI abnormalities in all types of CP surpassed 90%. MRI provides detailed information regarding structural brain abnormalities and is instrumental in identifying abnormalities that are relevant to pathological mechanisms.
In the context of CP, the age at which MRI is conducted varies considerably, with no consensus for addressing the issue of incomplete myelination. A preference seems to exist for including MRIs conducted at younger ages, even those performed during the neonatal period, as a predictive measure for CP [16].
CP cannot be definitively diagnosed during infancy. Traditionally, the diagnosis was made between 12 and 24 months of age. However, it can now be assigned before a corrected age of 6 months if motor dysfunction is observed in conjunction with abnormal neuroimaging and risk factor findings. Consequently, the term “high risk of CP” is employed to facilitate early specific intervention [17]. In the present study, MRI was performed at an average age of 4.37 years in severe CP cases and 5.12 years in mild to moderate CP cases. In another study, MRI examinations were performed at a mean age of 1.73 years, and even within the first 30 days of life [18]. This discrepancy is attributed to late referrals to tertiary centers and the waiting time required for imaging evaluations.
1. Clinical findings
This study reveals that the most common topographical classification of CP, both in mild-to-moderate and severe cases, is spastic quadriplegia. This finding aligns closely with the research of Khan et al. [4], who reported a 53.9% occurrence rate for the quadriplegic type. In contrast, Icagasioglu et al. [19] found the diplegic type to be the most common form of CP, accounting for 54.3% of cases, followed by the quadriplegic type at 34.3%. In their study, 30% of CP cases were classified as severe, with the remainder falling into the mild to moderate category [19]. However, Robinson et al. [15] proposed that the hemiplegia type was the most common, at 33.5%, followed by the diplegic type at 28.5%. Functional severity was assessed as either mild-to-moderate or severe, based on the GMFCS scale. The most frequently observed GMFCS levels were 1 and 2, accounting for 28.5% and 28.1% of cases, respectively [15]. In the present study, 50% of the participants were classified as GMFCS level 4 or 5. A small number of patients had a history of head trauma, which typically occurs incidentally during episodes of seizure, gait disturbance, or movement disorder over the course of the disease.
Patients with CP have high rates of comorbid epilepsy, one of the most common neurological disorders to co-occur with CP. In this study, the prevalence of epilepsy among patients with severe CP was 66.7%, while it was 43.3% in those with mild to moderate CP. On average, these figures are higher than others reported in the literature (30% to 49.4%), which may be due to the selection of cases. The rate of epilepsy was higher in severe CP than in mild to moderate cases, although this difference was not statistically significant. Despite ongoing debates in the literature about the relevance of epilepsy in individuals with CP, it is crucial to pay particular attention to the early identification and treatment of this comorbidity [20]. Yulianti et al. [8] posits that the prevalence of CP with epilepsy is higher than that of the condition without epilepsy. This suggests a close relationship between these disorders, with brain lesions often serving as the underlying cause of CP [8].
In the present study, although not a statistically significant finding, 71.6% of participants were found to be malnourished. Impairments in motor development and difficulties with feeding may particularly contribute to impacted growth. At the time of imaging, microcephaly was also found in approximately 60% of the severe CP group and 46% of the mild to moderate CP group. The incidence of preterm birth and low birth weight appears to be inversely proportional to head circumference. This study lacked data on head circumference at birth, as most of the patients were referred from other healthcare facilities. Abnormalities in head circumference in CP may occur in the postnatal period, potentially caused by the loss of brain mass due to brain disorders during development [8].
2. MRI findings
In most instances, CP arises from an injury to the fetal or neonatal brain. The timing and development of the brain are closely linked to the pathogenic patterns. If the abnormalities are primarily in the white or gray matter, injuries typically occur during the processes of synapse formation, dendritic growth, and the initiation of myelination beyond 20 weeks [16].
3. Gray matter injury
Injuries to the cortical area, basal ganglia, thalamus, or diencephalon can produce gray matter lesions. In the present study, approximately 46.7% of patients with severe CP and 16.7% of those with mild to moderate CP exhibited the gray matter type. This finding considerably exceeds the results reported by Kollu et al. [21], who identified only 9.5% of CP injuries in the superficial gray matter (cortical and subcortical) or deep gray matter (basal ganglia, thalami, and midbrain). Deep gray matter involvement impacting the putamen and globus pallidus has been associated with a mixed or non-spastic motor type, especially when cortical-subcortical involvement is not generalized [11]. Robinson et al. [15] identified a similar pattern in approximately 14.3% of the patients in their study. Khan et al. [4] reported encephalomalacia due to hypoxic-ischemic encephalopathy in 40.5% of participants. According to Choi et al. [13], gray matter injury is linked with hypoxic ischemia and kernicterus patterns. Children exhibiting a kernicterus pattern demonstrate a broad spectrum of clinical outcomes, ranging from mild-to-moderate to severe, and from hearing impairment to intellectual disability and epilepsy [13]. The discrepancies in results among researchers could be attributed to varying classification schemes. Superficial gray matter injury has been associated with both preterm and term births, while deep gray matter injury appears to be more common in term infants [21].
This study revealed a significant relationship between the severity of CP and gray matter injury. However, due to the limited sample size, this finding requires further validation through multiple studies. Reid et al. [22] suggests that the involvement of the globus pallidus is closely associated with gross motor function. The globus pallidus plays a role in both indirect and direct pathways with the putamen, coordinating muscle movement [22]. Choi et al. [13] also proposed a link between the severity of CP and hypoxic ischemic encephalopathy, with some post-kernicterus patients experiencing non-ambulatory CP.
4. White matter lesion
White matter injury (WMI), a common MRI abnormality associated with CP, appears in approximately 30% to 40% of cases [23]. In this study, approximately 26.7% of patients with severe CP and 33.3% of mild to moderate cases exhibited white matter lesions. Numerous studies of CP have frequently reported data on WMI, particularly in cases of spastic diplegia and quadriplegia. PVL, a specific type of white matter abnormality indicative of white matter damage, is also commonly found in patients with CP who were born prematurely [23]. This damage is characterized by signal abnormalities and/or volume loss in the periventricular and/or deep white matter. Additional characteristics of WMI may include scalloping of the ventricles, ventricular dilatation, or the presence of periventricular cysts [15]. This type of damage is believed to occur before week 34 of gestation. Furthermore, an MRI pattern frequently observed in CP is that of periventricular white matter (PWM) lesion, which is found in as many as 83% of patients. Reportedly, approximately 20% of PWM cases occur in term infants who exhibit spastic diplegia with PVL. These infants were also found to have a unilateral spastic type with focal periventricular gliosis.
5. Vascular insult
In this study, 16.7% of patients exhibited an MRI pattern indicative of vascular insult in cases of mild to moderate CP, while 10% did so in cases of severe CP. These percentages exceed those reported by Kollu et al. [21] (6.3%) and Towsley et al. [24] (8.7%). MRI may reveal a signal change, volume loss, or porencephaly within a defined vascular territory, with or without additional focal ischemic or hemorrhagic lesions [15]. Focal vascular insult is most commonly associated with spastic hemiplegic CP, in which the territory of the middle cerebral artery is more frequently affected.
6. Brain malformation
The findings revealed that the pattern of brain malformation was discernible on MRI in 30% of patients with mild to moderate CP and in 13.3% of patients with severe CP. These percentages are higher than those reported in studies conducted by Sharma and Dhande [25] and Bax et al. [26]. Brain malformation can manifest in cases of spastic diplegia with a history of preterm birth (13.6%), but it can also present as hydrocephalus, lissencephaly, pachygyria, polymicrogyria, or corpus callosal hypoplasia [25,26]. The research of Robinson et al. [15] indicated that brain malformations were more likely to be observed in children born at term and were associated with more severe motor disabilities. These pathologies typically develop during the first and second trimesters, a period characterized by the predominant occurrence of cortical neurogenesis. This process involves the proliferation, migration, and organization of neuronal precursor cells, followed by neuronal cells [16].
7. Normal
No specific morphological abnormalities were identified in one patient within each group. Given that CP severity is not strongly correlated with MRI normality, it appears warranted to maintain continuous follow-up with such patients through more advanced imaging modalities. Additionally, serious consideration should be given to metabolic and genetic testing in children who present with normal imaging results [18].
The limitations of this study include the fact that not all patients with CP can undergo MRI due to the high cost of this modality. Furthermore, the MRI device used in this study had a magnetic strength of 1.5 T, while devices in more developed countries can reach 3 to 7 T. This discrepancy could potentially impact the accuracy of MRI interpretation, particularly in cases of WMI. Another limitation is the lack of data on head circumference at birth. Many of our patients did not know this information, and they were often referred from other health facilities that may not have recorded these data. Additionally, we were unable to provide cognitive data from intelligence tests, as most members of our sample were unable to complete the intelligence quotient test due to their age. However, a strength of this study is that despite the often unclear timing of brain injury, the findings from the MRI interpretation demonstrate that MRI is an extremely valuable tool for identifying pathogenesis in cases of antenatal, perinatal, and postnatal damage.
In conclusion, most children with CP exhibit abnormal results on brain MRI. The most frequently observed abnormalities are lesions in the white and gray matter of the brain. The use of MRI is crucial in understanding the pathological basis of these brain abnormalities in CP.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: PIG, RN, and SMS. Data curation: PIG. Formal analysis: PIG, RN, and SMS. Methodology: PIG, RN, and SMS. Project administration: PIG, RN, and SMS. Visualization: PIG. Writing-original draft: PIG, RN, and SMS. Writing-review & editing: PIG, RN, and SMS.
Acknowledgements
The authors would like to thank the pediatric residents of Dr. Soetomo Hospital for their assistance with data collection.