Acute Necrotizing Encephalopathy in Children with COVID-19 Accompanied by Multisystem Inflammatory Syndrome
Article information
Several reports in both adults and children have demonstrated an association between coronavirus disease 2019 (COVID-19) and a variety of central and peripheral neurological insults. These can vary from mild symptoms, such as headaches and anosmia, to severe conditions like stroke, seizures, and encephalopathy [1]. Furthermore, instances of acute necrotizing encephalopathy (ANE) have been documented among severe COVID-19 cases in adults, along with a handful in the pediatric population [2]. ANE is an unusual form of encephalopathy, characterized by bilateral symmetrical lesions primarily found in the thalami and brain stem in infants and children [3].
Multisystem inflammatory syndrome in children (MIS-C) is a newly identified clinical syndrome associated with COVID-19. It is characterized by potentially severe inflammation and bears similarities to Kawasaki disease, and it can occur in children [4]. The clinical manifestations of MIS-C are diverse, including gastrointestinal symptoms, cardiac disease, mild or absent respiratory symptoms, and variable incidence of rash, red eyes, and changes to the oral mucous membrane [4]. In this report, we present two cases of COVID-19–associated ANE accompanied by MIS-C. To our knowledge, this study presents the first reported cases of COVID-19–associated ANE in the pediatric population of Korea.
1. Case 1
A previously healthy 6-year-old girl was admitted to a tertiary university hospital with a history of three seizure episodes, each lasting approximately 2 minutes. The girl also had abdominal pain, vomiting, and a fever that had persisted for 2 days. Upon her arrival in the emergency room, she had another seizure, which lasted for 5 minutes. This was managed with the administration of intravenous lorazepam at a dosage of 0.1 mg/kg. Subsequently, the patient was promptly admitted to the pediatric intensive care unit.
The patient presented in a drowsy state, with a Glasgow Coma Scale (GCS) score of 14 (eye opening 4, verbal response 4, motor response 6). Initial laboratory tests revealed leucopenia, with a count of 2.83 cells (×103/µL) composed of 40% lymphocytes and 57% neutrophils. The patient’s hemoglobin level was 13.0 g/dL, and she had a low platelet count of 107 (×103 cells/µL). Her C-reactive protein level was 0.82 mg/dL, procalcitonin level was 0.17 ng/mL, and D-dimer level was 6.87 µg/mL (normal range, 0 to 0.5). The measured prothrombin time and activated partial thromboplastin time were within the normal range. The patient’s aspartate transaminase and alanine transaminase levels were slightly elevated, at 122 IU/L (normal range, 5 to 40) and 47 IU/L (normal range, 5 to 41), respectively. Her levels of creatinine kinase, creatinine kinase-myocardial band, troponin I, and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) were also elevated, at 5,811 IU/L (normal range, 20 to 180), 37.7 ng/mL (normal range, 0 to 5), 0.45 ng/mL (normal range, 0 to 0.16), and 214 pg/mL (normal range, 0 to 153), respectively. A nasopharyngeal swab evaluated by polymerase chain reaction tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Cerebrospinal fluid (CSF) analysis indicated protein and glucose levels of 32.1 mg/dL (normal range, 12.0 to 60.0) and 70 mg/dL (normal range, 45 to 80; serum glucose level, 124 mg/dL), respectively, with normal CSF pressure and white blood cell (WBC) count. Magnetic resonance imaging (MRI) of the brain revealed bilateral symmetrical hypointense signals on T1-weighted images and hyperintense signals on T2-weighted images of the thalamus, midbrain, and pons; additionally, petechial hemorrhage was observed in the bilateral thalamus and pons (Fig. 1A and B). Electroencephalography and transthoracic echocardiogram results were normal. A next-generation sequencing panel for hereditary epilepsy revealed a negative result for mutations.

Brain magnetic resonance imaging showing bilateral symmetrical hyperintense signals on T2-weighted images of the thalamus, midbrain, and pons and petechial hemorrhage in the bilateral thalamus and pons (A, B). After steroid pulse therapy, the edematous change nearly resolved, but an increase was observed in residual hemorrhage in the bilateral thalamus, midbrain, and pons (C, D).
The patient was initially given intravenous methylprednisolone, which was tapered over a 3-week period. This was administered alongside antibiotic therapy (ceftriaxone at a dosage of 50 mg/kg every 12 hours and vancomycin at a dosage of 15 mg/kg every 6 hours) and antiviral therapy (acyclovir at a dosage of 15 mg/kg every 8 hours). The initial dose of methylprednisolone was 30 mg/kg/day. Three days after administering the starting dosage, the dose was halved every 2 days.
The patient’s neurological condition progressively improved. By the 7th day of hospitalization, she had regained the ability to walk slowly and was transferred to the general ward. On day 15 of hospitalization, she was discharged with no persistent neurological deficits. A brain MRI conducted 1 month later revealed an improved state of ANE, with virtually no edematous change. However, an increase was seen in residual hemorrhage in the bilateral thalamus, midbrain, and pons (Fig. 1C and D).
2. Case 2
A previously healthy 7-year-old girl was admitted to a tertiary university hospital with an altered mental state, abdominal pain, vomiting, and fever that had persisted for 1 day. Upon arrival at the intensive care unit, the patient was in a stupor, with a GCS score of 6 (eye opening 2, verbal response 2, motor response 4). Three hours into hospitalization, her blood pressure fell to a systolic blood pressure of 76 mm Hg and a diastolic blood pressure of 44 mm Hg. However, after 1 hour of hydration, her blood pressure returned to normal.
Upon admission, laboratory tests revealed a WBC count of 2.56 cells (×103/µL) with 10.1% lymphocytes and 84.5% neutrophils. The patient’s hemoglobin level was 12.3 g/dL, and her platelet count was low, at 109 (×103 cells/µL). Her C-reactive protein level was 1.09 mg/dL, procalcitonin level was 27.20 ng/mL, and D-dimer level was 13.74 µg/mL (normal range, 0 to 0.5). Both prothrombin time and activated partial thromboplastin time were prolonged, measuring 33.6 seconds (normal range, 11.0 to 15.0) and 103.6 seconds (normal range, 29.0 to 44.0), respectively. The patient’s fibrinogen and antithrombin III levels were below the normal range, at 183 mg/dL (normal range, 200 to 400) and 79% (normal range, 80% to 120%), respectively. Her aspartate transaminase and alanine transaminase levels were elevated, at 457 IU/L (normal range, 5 to 40) and 157 IU/L (normal range, 5 to 41), respectively. The patient’s creatinine kinase, creatinine kinase-myocardial band, troponin I, and NT-proBNP levels were elevated, at 1,091 IU/L (normal range, 20 to 180), 9.5 ng/mL (normal range, 0 to 5), 0.75 ng/mL (normal range, 0 to 0.16), and 1,031 pg/mL (normal range, 0 to 153), respectively. A nasopharyngeal swab evaluated by polymerase chain reaction tested positive for SARS-CoV-2. CSF analysis indicated protein and glucose levels of 56 mg/dL (normal range, 12.0 to 60.0) and 20.9 mg/dL (normal range, 45 to 80; serum glucose level, 72 mg/mL), respectively, with normal CSF pressure and WBC count. A brain MRI revealed severe bilateral symmetrical edema in the thalamus with diffusion restriction and hemorrhage in the central portion, as well as edema in the subinsular white matter, posterior basal ganglia, posterior limb of the internal capsule, upper midbrain, and pons. Focal diffusion restriction was also observed in the subinsular white matter, and multifocal small T2 and fluid-attenuated inversion recovery high-signal-intensity lesions were noted in the subcortical and deep white matter of both cerebral hemispheres (Fig. 2A and B). Electroencephalography results were moderately abnormal due to intermittent diffusion of high-amplitude 1.5 to 2 Hz delta activities in the background, suggesting diffuse cerebral dysfunction. A transthoracic echocardiogram was normal except for mild tricuspid and mitral regurgitation. A next-generation sequencing panel for hereditary epilepsy showed no mutations.
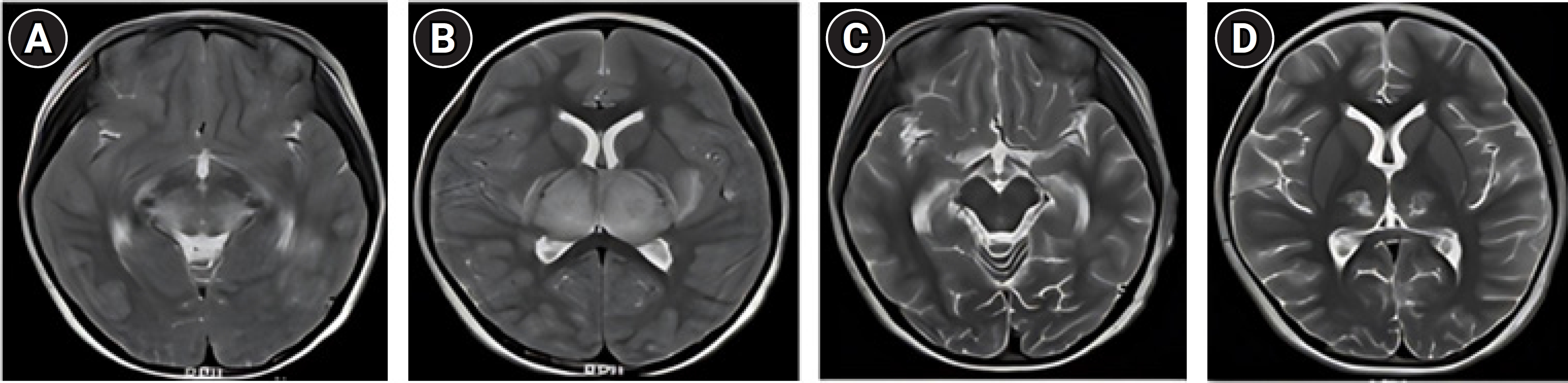
Brain magnetic resonance imaging showing severe bilateral symmetrical edema in the bilateral thalamus with diffusion restriction and hemorrhage in the central portion. Additionally, edema is evident in the subinsular white matter, posterior basal ganglia, posterior limb of the internal capsule, upper midbrain, and pons (A, B). After intravenous immunoglobulin and steroid pulse therapy, a markedly decreased extent of edema was observed in the bilateral thalamus, cerebral peduncle, subinsular white matter, claustrum, external capsule, posterior basal ganglia, posterior limb of the internal capsule, and dorsal pons (C, D).
The patient was initially administered intravenous immunoglobulins (IVIG; 2 g/kg/day for 1 day) and steroid pulse therapy, in addition to antibiotics (meropenem 40 mg/kg/dose every 8 hours and vancomycin 15 mg/kg/dose every 6 hours). On day 3 of hospitalization, the patient experienced seizures, which were controlled after approximately 10 minutes using lorazepam (0.1 mg/kg) and phenytoin (20 mg/kg/dose). On day 12, the patient was transferred to the general ward. Regarding mental status, she was nearly alert, with a GCS score of 12 (eye opening 4, verbal response 2, motor response 6). Her motor strength in both the upper and lower extremities had improved to grade 3, and her sensory functions were intact. Rigidity was observed in all four limbs. Although unable to speak, she demonstrated comprehension of most auditory input.
On day 21 of hospitalization, the patient was transferred to the department of rehabilitation medicine. After receiving rehabilitation treatment, she was discharged on day 46 of her hospital stay. At discharge, her consonant and vowel accuracy while speaking had improved. Furthermore, she was able to walk independently; however, continuous bilateral tremors of the upper and lower extremities were observed.
A brain MRI performed 1 month after discharge revealed a marked decrease in edema within the thalamus, cerebral peduncle, subinsular white matter, claustrum, external capsule, posterior basal ganglia, posterior limb of the internal capsule, and dorsal pons. Hemorrhage was noted in both the thalamus and cerebral peduncle, along with newly apparent multifocal petechial hemorrhage in the pons, both cerebral hemispheres, and both basal ganglia (Fig. 2C and D). These findings were considered indicative of an improved state of ANE.
The patients in both cases exhibited multi-organ dysfunction, including encephalopathy, liver dysfunction, coagulopathy, and cardiovascular involvement. They also showed signs of a cytokine storm, meeting the World Health Organization case definition of MIS-C [5]. Our search of the literature identified five cases of COVID-19–associated ANE [2,6-9]. The ages of these patients ranged from 5 weeks to 11 years. The primary symptoms presented were seizures, altered mental status, motor weakness, and speech disturbances. All patients received treatment with high-dose steroids and/or IVIG. Two of the five patients were also treated with tocilizumab [2,8], and one underwent plasmapheresis [7]. The neurological examination was reported as normal for one patient [7], while three patients had residual neurological deficits [2,8,9], and one patient died [6]. In the present two cases of COVID-19–associated ANE, both patients were treated with high-dose steroids and/or IVIG. Upon discharge, the neurological examination of one patient was normal, while the other exhibited neurological deficits. Despite the rarity of ANE reports, as demonstrated in these cases, any patient presenting with seizures or altered mental status should be considered for potential COVID-19–associated ANE to ensure prompt diagnosis and treatment.
This study received approval from the Research Ethics Committee of Inha University Hospital (approval number: 2023-04-035). Due to the retrospective nature of the study, the requirement for written informed consent was waived.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: DSP, DHK, and YSK. Data curation: DSP and SWY. Formal analysis: DSP, DHK, and YSK. Methodology: DSP, DHK, and YSK. Project administration: DSP and YSK. Visualization: DSP, DHK, and YSK. Writing-original draft: DSP. Writing-review & editing: YSK.
