Real-Life Efficacy and Tolerability of Lacosamide in Pediatric Patients Aged 4 Years or Older with Drug-Resistant Epilepsy
Article information
Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of adjunctive lacosamide therapy in pediatric patients aged ≥4 years with drug-resistant epilepsy (DRE).
Methods
Medical records of children aged 4 to 19 years treated with lacosamide as adjunctive therapy for DRE were retrospectively reviewed. The patients were divided into two groups according to their age at the start of lacosamide treatment: group A (aged 4–15 years) and group B (aged 16–19 years). Changes in seizure frequency from baseline, adverse events, and the retention rate were evaluated at each follow-up visit.
Results
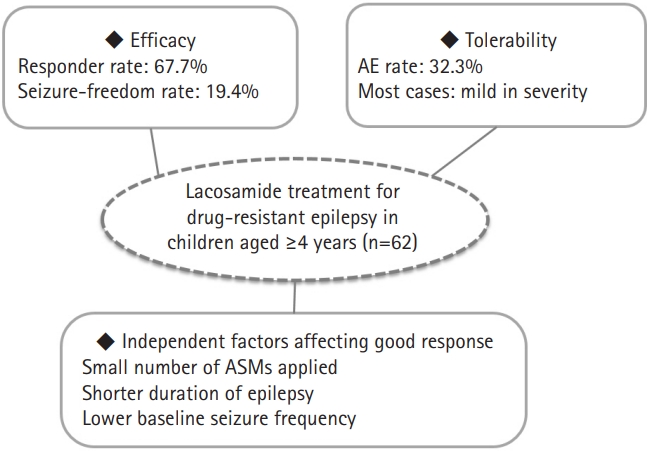
Sixty-two patients (33 males and 29 females) with a mean age of 11.4 years (range, 4 to 19) were included. The mean duration of follow-up was 20.1±12.9 months. The mean maintenance dose of lacosamide was 6.7±4.8 mg/kg/day. Forty-two patients (67.7%) were responders (≥50% reduction in seizures) with 19.4% (12/62) achieving freedom from seizures. The response rate did not differ significantly between groups A and B (67.6% vs. 68.0%, P=0.795) and was not affected by the concomitant use of sodium channel blockers. Significant independent factors associated with a good response to lacosamide treatment were a shorter duration of epilepsy (P=0.035) and fewer concomitant anti-seizure medications (P=0.002). Mild transient adverse events were observed in 20 patients (32.3%).
Conclusion
Lacosamide adjunctive therapy was efficacious and tolerated in children aged ≥4 years with DRE. Early use of lacosamide may be helpful for a good response to drug-resistant seizures.
Introduction
Approximately 25% to 30% of children with epilepsy have drug-resistant seizures or experience significant adverse events (AEs), despite the introduction of multiple new anti-seizure medications (ASMs) over the past 20 years [1]. Such drug-resistant epilepsy (DRE) leads to prominent risks of neuronal damage and cognitive decline in these patients; therefore, novel, effective, and well tolerated ASM therapies are urgently required to improve treatment outcomes. Lacosamide (LCM) is an ASM that exerts anticonvulsant activity by selectively enhancing slow inactivation of voltage-gated sodium channels [2]. LCM has high oral absorption with linear pharmacokinetics, low protein binding, good renal clearance, and low potential for drug-drug interactions [3]. LCM was approved for the treatment of focal seizures in patients aged ≥4 years in the United States and European Union in 2017.
In adults, several randomized controlled trials have demonstrated the efficacy and tolerability of LCM as adjunctive therapy and monotherapy for uncontrolled focal seizures [4,5], with further support from experience in clinical practice [6]. However, the effectiveness of adjunctive LCM in children and adolescents has been investigated in a few observational [7-14] or prospective studies [15,16] and only one double-blind randomized controlled trial (Supplementary Table 1) [17]. Among these studies, only three were conducted in Asia [9,10,13], and only two other studies included patients with general seizures and focal seizures [11,16].
Herein, we present our experience with adjunctive LCM therapy in pediatric patients aged ≥4 years with DRE at a single tertiary center. To the best of our knowledge, this is the largest long-term study of a pediatric population in East Asia.
Materials and Methods
1. Patients
In this retrospective cohort study, we retrospectively reviewed the electronic medical records of patients treated with oral LCM as an adjunctive treatment for focal epilepsy at Pusan National University Children’s Hospital between May 2018 and April 2022. Patients were selected based on the following criteria: (1) ≥4 years to <20 years of age; (2) being affected by drug-resistant seizures; (3) exhibiting at least one seizure per month during the 6 months before LCM was administered; and (4) concomitant ASMs being unchanged for the duration of the study. Patients with progressive neurological disorders and those with insufficient medical records were excluded from the study.
2. Data collection and evaluation for treatment outcomes of LCM
The following data were collected: sex; seizure types; presence of intellectual disability, etiology of epilepsy; history of ketogenic diet, vagus nerve stimulation (VNS), or epilepsy surgery; age at seizure onset; age at initiation of ASM use; age at initiation of LCM use; duration of epilepsy; duration of LCM treatment; number of ASMs previously administered; monthly seizure frequency; initial daily dose of LCM; final maintenance dose of LCM; retention at the end of the study; and AEs. The optimal maintenance dose of LCM was determined for each patient, depending on the clinical response and tolerability. Seizure types were classified as generalized, focal, combined focal, and generalized. Epilepsy etiologies were classified into genetic, metabolic, infectious, structural, and unknown based on the new classification of seizures and epilepsy by the International League Against Epilepsy (2022).
The response to LCM treatment was assessed based on the mean monthly seizure frequency during the follow-up period over the last 6 months. It was classified as seizure-free (100% reduction), 50%–99% reduction, 1%–49% reduction, and no change in monthly seizure frequency. A good response was defined as ≥50% reduction (seizure-free or 50% to 99% reduction). Patients with a good response were considered responders, and all patients with a <50% reduction in seizure frequency were designated as non-responders. The patients were divided into two groups according to age at the start of LCM: group A (4–15 years of age) and group B (16–19 years of age). We obtained information on changes in seizure frequency compared with baseline, AEs, and discontinuation and retention rates at each follow-up visit.
3. Statistics
All analyses were performed using R software version 3.2.1 meta package (R Foundation for Statistical Computing, Beijing, China), and all statistical tests were two-sided. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to evaluate the effect of ASMs on all dichotomous outcomes. Multivariate logistic regression was used to identify factors independently associated with a good response to LCM treatment. ORs with 95% CIs were used to test for differences within groups. In all analyses, P values of <0.05 indicated statistical significance.
4. Standard protocol approvals and patient consent
Ethical approval for this study was provided by the Institutional Review Board of Pusan National University Yangsan Hospital (number: 05-2023-038). Informed consent was obtained from all the participants.
Results
1. Demographic and clinical profile of the patients
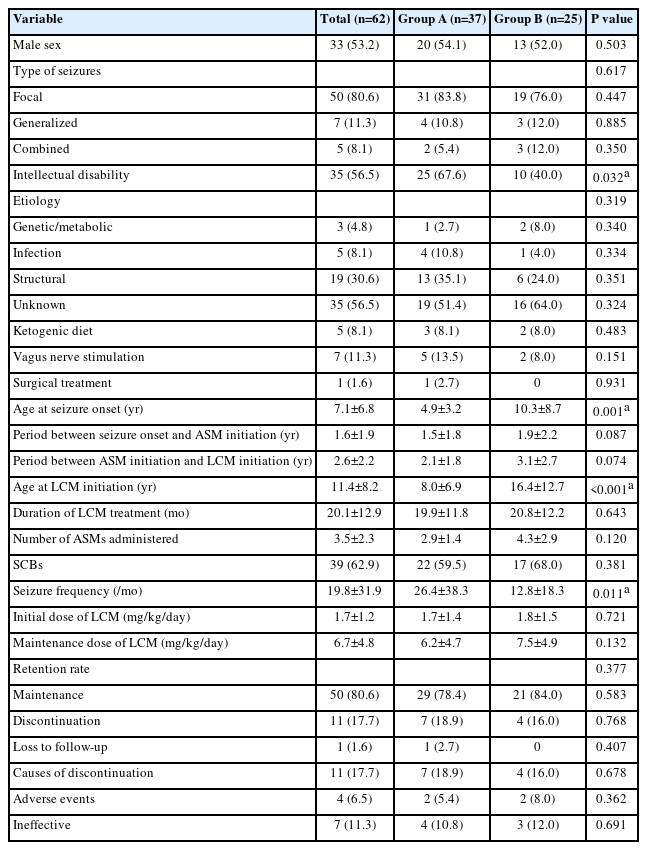
This study recruited 62 children and adolescents, comprising 33 males and 29 females with ages ranging between 4 and 19 years (mean, 11.4±8.2) at the initiation of LCM treatment (Table 1). The mean age at the first seizure was 7.1 years (range, 2.8 to 13.8), and the mean period between seizure onset and ASM initiation was 1.6 years (range, 0.3 to 5.9). The mean seizure frequency was 19.8 per month (range, 2 to 66). The starting dosage of LCM was 1.7±1.2 mg/kg/day, and the final maintenance dosage was 6.7±4.8 mg/kg/day. The retention rate at the end of the study was 80.6%.
Comparison of demographic profiles and clinical features between groups A (aged 4–15 years) and B (aged 16–19 years), according to age at lacosamide initiation
Table 1 presents the demographic characteristics and clinical features of groups A and B when they were started on LCM. There were no significant differences between groups A and B in the sex ratio (male ratio, 54.1% vs. 52.0%, P=0.503); seizure type; etiologies; history of ketogenic diet (8.1% vs. 8.0%, P=0.483), VNS (13.5% vs. 8.0%, P=0.151), epilepsy surgery (2.7% vs. 0.0%, P=0.931); period between seizure onset and ASM initiation (1.5±1.8 years vs. 1.9±2.2 years, P=0.087); period between ASM initiation and starting LCM (2.1±1.8 years vs. 3.1±2.7 years, P=0.074), duration of LCM treatment (19.9±11.8 months vs. 20.8±12.2 months, P=0.643), number of ASMs previously administered (2.9±1.4 vs. 4.3±2.9, P=0.120), initial daily dose of LCM (1.7±1.4 mg/kg vs. 1.8±1.5 mg/kg, P=0.721); daily maintenance dose of LCM (6.2±4.7 mg/kg vs. 7.5±4.9 mg/kg, P=0.132); or the retention rate (78.4% vs. 84.0%, P=0.583). In contrast, the patients in group A had a higher proportion of intellectual disability (67.6% vs. 40.0%, P=0.032) and a higher baseline monthly seizure frequency (26.4±38.3 vs. 12.8±18.3, P=0.011) than those in group B. The mean ages at seizure onset (4.9±3.2 years vs. 10.3±8.7 years, P=0.001) and LCM initiation (8.0±6.9 years vs. 16.4±12.7 years, P<0.001) were significantly lower in group A patients than those in group B (Table 1).
2. Efficacy
Of the 62 patients, the proportions of patients who were seizure-free (100% reduction), experienced a 50% to 99% reduction in seizure frequency, and were classified as responders (≥50% reduction) were 19.4% (12/62), 48.4% (30/62), and 67.7% (42/62), respectively (Fig. 1). The response rate did not differ significantly between groups A and B (67.6% vs. 68.0%, P=0.795). Thirty-nine patients (62.9%) used sodium channel blockers (SCBs; carbamazepine, lamotrigine, oxcarbazepine, or phenytoin) as part of combination therapy. There was no significant difference in the response rate between patients who did and did not receive concomitant SCBs (64.8% vs. 70.3%, P=0.597) (Fig. 2).
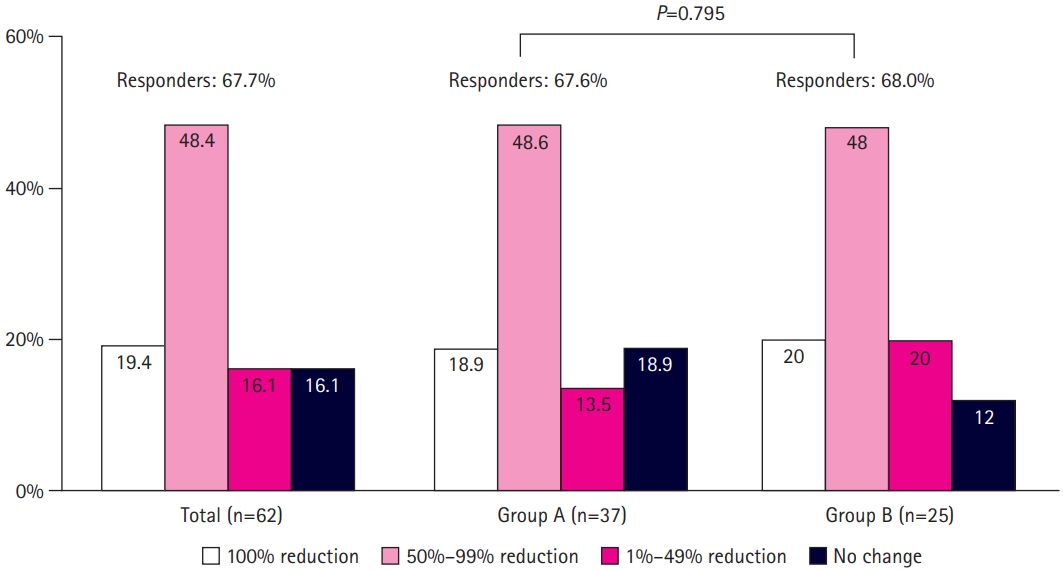
Reduction in seizure frequency and response rate to lacosamide in group A (aged 4–15 years) and group B (aged 16–19 years) (P=0.795, group A vs. group B).
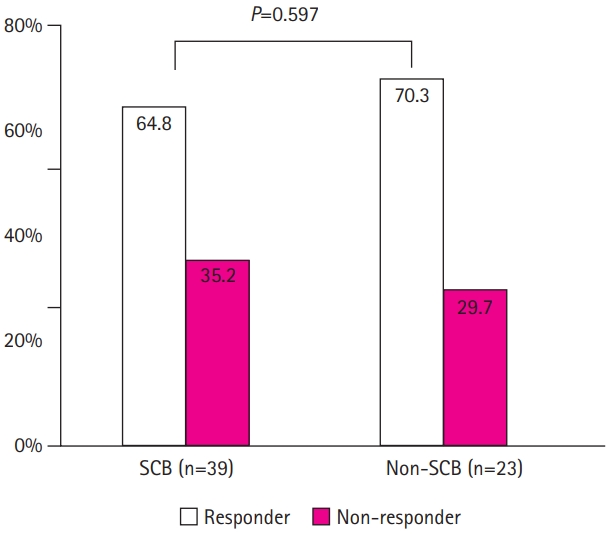
Comparison of the response rate to lacosamide according to type of concomitant anti-seizure medication at baseline (P=0.597, sodium channel blocker [SCB] vs. non-SCB).
The demographic and outcome data of responders and non-responders were investigated and analyzed (Table 2). The responders were significantly older at seizure onset (7.9±5.7 years vs. 5.6±4.5 years, P=0.038) and ASM initiation (9.4±5.3 years vs. 7.4±5.1 years, P=0.041) than the non-responders. The responders had a significantly shorter duration of epilepsy (1.8±1.2 years vs. 4.4±2.9 years, P=0.015) and a significantly longer duration of LCM treatment (17.8±9.3 months vs. 10.7±6.9 months, P=0.039) than the non-responders. The proportion of patients with intellectual disability (47.6% vs. 75.0%, P=0.042) and the number of ASMs administered (2.8±1.6 vs. 4.9±2.3, P=0.006) were conspicuously lower in the responders than in the non-responders. The responders showed a significantly lower discontinuation rate (7.1% vs. 40.0%, P=0.002) than the non-responders. Ten patients with Lennox-Gastaut syndrome and four with sleep-related hypermotor epilepsy were classified as having epilepsy syndrome (Table 2). Of the 10 patients with Lennox-Gastaut syndrome, eight were responders, and two of them were seizure-free. All four patients with sleep-related hypermotor epilepsy were responders, and two of them remained seizure-free
In a logistic regression model of the independent significant factors affecting the seizure outcomes of adjunctive LCM therapy (Table 3), a good response (≥50% reduction) was significantly negatively correlated with the duration of epilepsy (P=0.035) and number of ASMs previously administered (P=0.002). The number of ASMs (P=0.013) and baseline seizure frequency (P=0.046) were significant factors that negatively affected the likelihood of freedom from seizures.
3. Safety and tolerability
At least one AE was reported in 20 patients (32.3%) (Table 4), and some AEs appeared simultaneously. The mean dosage at which AEs occurred was 11.5±10.2 mg/kg/day. Somnolence was the most common AE (12/62 [19.4%]), followed by dizziness (9.7%), nausea (6.5%), headache (4.8%), and anxiety/irritability (1.6%). There was no significant difference between groups A and B in the rate of AEs, dosage at AE appearance, or type of AE (Table 4). No severe or life-threatening AEs were reported in this study. All AEs were tolerable or resolved in time through dose reduction or LCM discontinuation. There were no significant laboratory anomalies in liver function, renal function, or hematological examinations.

Adverse events and profiles between groups A (aged 4–15 years) and B (aged 16–19 years) according to age at lacosamide initiation
LCM was discontinued at similar rates in groups A and B (18.9% vs. 16.0%, P=0.678) (Table 1). The rate of discontinuation due to AEs was not significantly different between the two groups (5.4% vs. 8.0%, P=0.362). There was no significant difference in the discontinuation rate according to whether patients did or did not receive SCBs (8/39 [20.5%] vs. 3/23 [13.0%], P=0.457; data not shown).
The efficacy, tolerability, and significant independent factors affecting a good response to LCM treatment in children and adolescents aged ≥4 years with DRE are summarized in Fig. 3.
Discussion
In this retrospective study, adjunctive LCM therapy was effective in reducing seizure frequency and was generally well tolerated in 62 children and adolescents (aged ≥4 to ≤19 years) with DRE. The rate of response (≥50% reduction in seizures) was 67.7% (42/62), with 19.4% (12/62) achieving freedom from seizures; this proportion did not differ significantly according to age (4–15 years vs. 16–19 years; 67.6% vs. 68.0%, P=0.795). The response rate of LCM was similar in both groups, regardless of whether a concomitant SCB was used (64.8% vs. 70.3%, P=0.597). Significant factors affecting a good response to seizure reduction were a shorter duration of epilepsy (P=0.035) and fewer ASMs previously administered (P=0.002). At least one AE was reported in 32.3% (20/62) of the patients. All the AEs were mild and transient, and no severe or life-threatening AEs were reported. To the best of our knowledge, this study is based on real-life clinical practice and reflects the efficacy and AEs of LCM in the largest population of pediatric patients with drug-resistant focal and/or generalized epilepsy studied to date, with the longest follow-up duration of LCM treatment (20.1±12.9 months), at a single tertiary center in East Asia.
LCM has been approved by the licensing authorities in the European Union and in the United States as monotherapy for focal seizures in patients ≥1 month of age and as add-on therapy for generalized seizures in patients ≥4 years of age. However, in Korea, LCM has been approved as an add-on therapy for focal seizures in patients aged >16 years. This has resulted in very little research on the outcomes of LCM treatment in pediatric patients. Approval for pediatric use only occurs with a substantial delay after promising results have been achieved in adults [18].
In adult trials, LCM demonstrated a noteworthy advantage in treating DRE, with 30% to 40% of patients achieving a ≥50% reduction in seizure frequency at doses of 400 to 600 mg/day [4,19]. Since 2010, a few studies have described similar benefits of LCM in children and young adults with DRE [7-15]. In these studies, the mean response rate for ≥50% seizure reduction ranged between 20% and 67%, and the seizure-free rate was 11% to 19%. In a prospective study of 21 pediatric patients with refractory epilepsy of various seizure types, LCM was demonstrated to be an effective ASM [16]. Interestingly, two patients with Lennox-Gastaut syndrome showed a >90% seizure reduction. Although our patients showed a similar age and maintenance dose of LCM compared to those in previous pediatric studies, the rate of responders in our study was 67.7%, which was higher than that in previous studies. The patients in our study showed fewer ASMs (3.5±2.3 vs. 3–7.2) and a shorter duration of epilepsy (2.6±2.2 years vs. 3–9 years) than those in previous studies [7-17]. This was consistent with the results of logistic regression analysis for significant factors affecting the seizure outcomes of adjunctive LCM therapy in our study. Therefore, even in children with DRE, LCM may be more effective in reducing seizures in patients who do not have a long duration of epilepsy or have not used a large number of ASMs. Taken together, this indicates that LCM could have a significant clinical impact on patients with a shorter duration of epilepsy in whom a small number of ASMs has failed. From a different perspective, it might be considered that LCM may elicit a better treatment response in patients with less severe DRE, because patients who have taken a large number of ASMs or have shown longer treatment duration could have a higher degree of intractability to the medication. However, it is difficult to infer this conclusively due to the limitations of our retrospective study.
Previous studies on adjunctive LCM therapy have shown better [20,21] or similar [22] tolerability profiles in adult patients not receiving concomitant SCBs than in those receiving SCBs, such as carbamazepine, oxcarbazepine, or phenytoin. It has been suggested that combinations of ASMs with different mechanisms of action might be more efficacious and/or well tolerated than combinations of ASMs with similar mechanisms of action. A retrospective cohort study of children and adolescents with focal, generalized, or mixed epilepsy (n=223) showed that the use of SCBs was an independent predictor of time to LCM treatment failure [23]. Additionally, analyses of pooled data from double-blind placebo-controlled trials in adults showed a potential for better tolerability of adjunctive LCM when taken without SCBs [24]. However, LCM was efficacious regardless of whether SCBs were part of the concomitant ASM regimen in our study (64.8% vs. 70.3%, P=0.597) (Fig. 2). Unlike other SCBs, LCM does not alter the fast inactivation of voltage-gated sodium channels; instead, it selectively enhances the slow inactivation of voltage-gated sodium channels, thereby increasing the proportion of sodium channels unavailable for depolarization [2]. LCM has a predictable pharmacokinetic profile with high oral bioavailability, minimal protein binding (<15% to 19%), low potential for drug-drug interactions, and good renal clearance [3]. Moreover, LCM has not been shown to induce or inhibit cytochrome P450 enzymes in preclinical and clinical studies [25]. Due to the above differences in mechanisms, adjunctive LCM therapy might be more effective in reducing seizures, even if previous SCBs led to a lower response in terms of seizure reduction. Further prospective investigations of combination treatments with SCBs in a larger number of children are needed.
Multiple clinical pharmacology trials have demonstrated that LCM has favorable characteristics compared to other ASMs [3,25]. It is rapidly absorbed after oral administration, with maximum plasma concentration being reached 0.5 to 4 hours after intake. The pharmacokinetics are linear and dose-proportional, with low inter- and intra-individual variability. A population pharmacokinetic analysis of LCM phase 3 trial data suggested that there were 15%–20% and 20%–30% lower LCM plasma concentrations in the presence of enzyme-inducing ASMs [26]. In children, as in adults, the reduction in plasma concentrations is modest. In our study, the LCM plasma level of each patient was not investigated during the study period; therefore, it was not possible to compare efficacy according to plasma drug levels. In clinical practice, ASMs are usually titrated based on individual efficacy and tolerability and not on concentration, and the maintenance dose of LCM was also determined for each patient depending on the clinical response and tolerability.
The AEs most commonly reported during adjunctive LCM therapy in children were similar to those reported during LCM treatment in adults (e.g., somnolence, dizziness, headache, nausea, and diplopia) [27,28]. Most AEs associated with LCM in adults are dose-related and reversible upon discontinuation or dose reduction [29]. The mean dose of LCM in our study was 11.5 mg/kg/day, and the incidence of AEs was 32.3%, which is similar to that reported in previous studies [12,14,16]. LCM was discontinued in four patients (6.5%) because of somnolence, dizziness, or severe irritability. None of our patients experienced severe to life-threatening AEs or aggravated seizure frequency. Among LCM-treated adults on SCB ASM, discontinuation due to AEs was dose-dependent (200 mg, 5.5%; 400 mg, 14.4%; and 600 mg, 31.0%) and most commonly occurred because of dizziness (7.0% of patients) [24]. In contrast, adjunctive LCM therapy was well tolerated in our pediatric patients, regardless of whether SCBs were part of the treatment regimen (discontinuation rate, 20.5% vs. 13.0%, P=0.457). Individualized titration and dosing could enable optimization of the tolerability of LCM add-on therapy in children administered various ASM combinations.
This study has some limitations. First, this was a retrospective study involving a rather small number of patients, although this study included the largest number of pediatric patients in East Asia analyzed to date. Second, the effect of dosage and serum concentration of other ASMs on the efficacy of LCM was not investigated. Third, we did not evaluate the effect of LCM on behavioral or neurocognitive outcomes in pediatric patients. Further prospective large-scale studies in young children or infants with DRE are needed to clarify the benefits of LCM in these groups.
In conclusion, our retrospective study demonstrated that adjunctive LCM therapy was efficacious and well tolerated in children aged ≥4 years with drug-resistant focal and/or generalized epilepsy. The response rate of LCM was similar in both groups, regardless of whether a concomitant SCB was used. Significant factors associated with a good response to adjunctive LCM therapy were a shorter duration of epilepsy and fewer ASMs applied. Therefore, early use of LCM may be helpful for a good response in children with DRE.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.26815/acn.2023.00073.
Previous studies on adjunctive lacosamide therapy in children and adolescents
Notes
Sang Ook Nam is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: SYB and YJL. Data curation: JK and SYL. Formal analysis: JK and SYL. Methodology: SYB, SON, and YJL. Project administration: YMK and GMY. Visualization: YMK and GMY. Writing-original draft: SYB and YJL. Writing-review & editing: SON, YMK, GMY, and YJL.
Acknowledgements
This study was supported by a 2-Year Research Grant of Pusan National University.