Clinical Findings of Sydenham Chorea in Pediatric Patients: A Single-Center Retrospective Study
Article information
Abstract
Purpose
Sydenham chorea is known for its rapid, irregular, and aimless involuntary movements and is considered a benign and self-limiting condition among the major manifestations of rheumatic fever. The current study reviewed the demographic, clinical, and paraclinical findings of pediatric patients with Sydenham chorea.
Methods
This cross-sectional study was conducted among 22 patients with Sydenham chorea who were admitted to the pediatric wards of Mashhad Imam Reza and Ghaem Hospitals between 2006 and 2016. Data from these patients’ medical records were extracted, organized using checklist forms, and analyzed.
Results
Eight patients were male and 14 were female. The average age was 10.09±3.53 years. In 31.8% of patients, chorea was the only sign of rheumatic fever. Chorea was unilateral in 21.1% of patients. The most common clinical findings were, in descending order, jerky movements, facial grimacing, gait disorders, mental disorders, speech disorders, muscle weakness, and milkmaid's grip. Cardiac auscultation was normal in 76.2% of patients, while a holosystolic murmur was heard in 23.8%. In laboratory exams, 50% of patients were erythrocyte sedimentation rate (ESR)-positive, 31.2% were C-reactive protein (CRP)-positive, and 53.3% were anti-streptolysin O (ASO)-positive. Echocardiography showed the prevalence of mitral regurgitation (63.6%), aortic regurgitation (45.5%), tricuspid regurgitation (22.7%), pulmonary regurgitation (4.5%), and pericardial effusion (4.5%).
Conclusion
This study showed that Sydenham chorea can be the only sign of rheumatic fever. This disease typically occurs in children between the ages of 7 and 12. ESR, CRP, and ASO can be the most effective laboratory tests for diagnosis.
Introduction
Rheumatic fever (RF) is among the complications that may develop from group A beta-hemolytic streptococcal (GABHS) infections, resulting from an autoimmune reaction [1]. This systemic autoimmune disease is a delayed result of infection with GABHS bacteria in the throat area, and most commonly occurs in children between the ages of 5 and 14 years [2,3]. Sydenham chorea is known for its rapid, irregular, and aimless involuntary movements—typically in the face, hands, and feet of the patients—and is considered a benign and self-limiting condition among the major manifestations of RF. Sydenham chorea, which occurs in 20% to 30% of children with RF, is considered a neurological manifestation of RF that shows itself approximately 6 months after the severe infection; however, it can also rarely be an initial sign of RF onset [2,4,5].
Chorea is identified based on its aimless, involuntary movements, muscle weakness and discordance, and mental disorders. These symptoms worsen when the patient is awake or under stress, and disappear during sleep. The entire body is affected, but it is more severe on the face and extremities. Speech may be affected and become explosive or pausing. Patients’ handwriting may be impaired, wavy, or spiky. Arm extension overhead may cause pronation in one or both hands. Irregular hand muscle contractions occur when the patient squeezes the examiner's hand, and when the patient moves the hands forward, the fingers become hyperextended. The discoordination can easily be observed when a patient is asked to button or unbutton a shirt. Mental instability is clearly visible; there is a tendency toward irritability, stubbornness, and resentment. A child's teacher may declare that the student is not focused, is anxious, negligent, and does not collaborate [6,7].
Sydenham chorea can be the only sign of RF and thus is sufficient, on its own, to diagnose RF. The diagnosis of Sydenham chorea's is entirely clinical, based on chorea's clinical signs, a history of RF, and excluding other causes [8,9]. Poverty, crowded communities, national country population, lack of access to high-quality public health, and fear of physicians prescribing penicillin due to allergic reactions are among the major factors associated with this disease's prevalence [6,10].
M-type streptococcal serotypes have been more frequently isolated than other streptococcal serotypes in patients with a history of RF; however, since the serotypes are unknown in clinical diagnosis of streptococcal pharyngitis, all group A serotypes need to be considered pathogenic and all infections effectively treated [7]. It is worth noting that not all rheumatogenic serotypes of group A streptococci are equally dangerous [11].
Common antibodies against group A hemolytic streptococci and caudate nucleus neurons are found in patients with Sydenham chorea. These antibodies, also known as anti-basal ganglia antibodies, cause disruptions in basal ganglia neurons. Central nervous system (CNS) vasculitis causes cell degeneration and results in edema in the endothelium of blood vessels, perivascular infiltration, and cerebral petechial hemorrhage [12-14]. In one study, disrupted cerebral circulation was stated as a cause of Sydenham chorea [15].
Genetics plays a critical role in the pathogenesis of Sydenham chorea. For instance, patients with Sydenham chorea have a higher frequency of D8/17 alloantigen expression. However, susceptibility to the disease is considered to be an interplay between genetic factors such as this and environmental ones [16].
Despite the low prevalence of RF in developed nations, it is still considered a major problem among children and teenagers in developing countries. Economic impacts caused by disabilities, neurological or mental disorders, or mortality due to RF lead to increased public health costs at regional and national levels. The results of this study can be used to better understand the damages caused by the disease and may change our operational perspective on the matter. Employing clinical and paraclinical findings of the disease would be helpful in early diagnosis, effective treatment of the patients, and prevention of its devastating impacts and increased public health costs.
Materials and Methods
All patients with Sydenham chorea admitted to the pediatric wards of Mashhad Imam Reza and Ghaem Hospitals between 2006 and 2016 were included. This study was conducted with a retrospective approach using patients' medical records. Data were extracted from the files on age, sex, initial complaint, clinical signs of RF (arthritis, carditis, subcutaneous nodule [SCN], erythema marginatum [ERM], and chorea), clinical signs of Sydenham chorea (walking disorder, pronator sign, muscular weakness, speech issues, lingual dyskinesia, facial grimacing, and mental signs), physical examination including cardiac auscultation, and laboratory results, as well as electrocardiography (ECG), electroencephalography (EEG), echocardiography, and brain magnetic resonance imaging (MRI) results. In cases where the patient information was incomplete (nine patients), additional information was obtained via telephone contact. The data were organized using checklist forms and then analyzed. In this study, the normal limits for the erythrocyte sedimentation rate (ESR) and anti-streptolysin O (ASO) were defined as 20 mm/hr and 200 IU/mL, respectively. Leukocytosis was defined as white blood cell (WBC) count >11,000/mm3, leukopenia as WBC count <3,700/mm3, thrombocytosis as platelet count > 500,000 μL, and anemia as hemoglobin <11 and <12 g/dL in children aged 5 years and under and children older than 5 years, respectively. Fever was defined as an oral temperature ≥37.8°C. The normal value for the ejection fraction was defined as being between 56% and 78%. Carditis was defined as a new pathological valvular lesion detected via echocardiography.
This study was approved by the medical ethics committee of Mashhad University of Medical Sciences (Ethics code: IR.MUMS.fm.REC.1396.722). All patients and their families were informed and signed consent forms. The information used in this study was gathered from an anonymized data bank, and thus no personal information on patients' identities has been revealed.
After completion of the checklist forms, the data were entered into SPSS version 10 (SPSS Inc., Chicago, IL, USA) and analyzed. The results were described in the form of tables, figures, and central and dispersion indexes.
The normality of the data distribution was checked using the Kolmogorov-Smirnov test. The t-test and Mann-Whitney U test were conducted in order to identify the relationships among quantitative variables for data with normal and non-normal distributions, respectively. The relationships among qualitative variables were determined using the chi-square and Fisher exact tests. Correlations were also examined using the Pearson test for normally distributed data and the Spearman test for non-normally distributed data. The statistical analysis was conducted using SPSS version 10 (SPSS Inc., Chicago, IL, USA), and a P<0.05 was considered to indicate statistical significance.
Patient and public involvement
Neither patients nor the public was involved in the design, implementation, reporting, or dissemination plans of this study.
Results
This study was conducted on 22 patients. The diagnoses of Sydenham chorea were made by pediatric neurologists based on clinical symptoms, history of RF, and the exclusion of other causes of chorea. Studied cases included eight boys (36.4%) and 14 girls (63.6%), with an average age of 10.09±3.53 years (range, 3 to 17). Fig. 1 illustrates the age distribution of patients included in this study.
The average weight of the patients at birth was 2.83±0.67 kg, and the average weight upon hospital admission was 30.29±10.93 kg. Seventeen patients (77.3%) were born vaginally, while five were born by cesarean section (22.7%). Among 22 patients, 20 were born full-term, one was born pre-term, and one was born post-term. Nine patients were the family's first child, seven patients were the second child, four patients were the third child, and two patients were the fourth child. Nine of the parents were relatives (cousins), and 13 were unrelated.
The reasons for referral to the hospital were chorea for 19 patients (86.4%), fever for two patients (9.1%), and arthritis for one patient (4.5%). In 31.8% of patients, chorea was the only sign of RF. Fifteen patients manifested both chorea and carditis, while no patients manifested arthritis, ERM, or SCN. Two of the studied patients had a history of arthritis (10 to 14 days prior to the onset of chorea), and one patient had a history of ERM (2 weeks before chorea). Chorea was bilateral in 78.9% of patients and unilateral in 21.1% of patients.
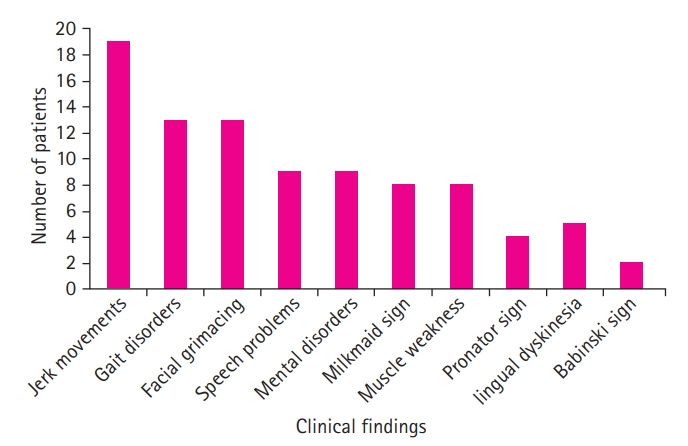
A study of the patients’ clinical findings showed the following significant signs of Sydenham chorea, in descending order of frequency: jerky movements (86.4%), facial grimacing (59%), gait disorders (59%), mental disorders (47.4%), speech problems (47.4%), muscle weakness (42.1%), milkmaid's grip (42.1%), lingual dyskinesia (26.3%), fever (22.7%), the pronator sign (21.1%), and the Babinski sign (9%). Fig. 2 shows the distribution of patients based on determined clinical findings.
Cardiac auscultation detected a holosystolic murmur in 23.8% of patients, while 76.2% had normal results. In laboratory examinations, 50% of patients were ESR-positive, 32.2% were C-reactive protein-positive, and 53.3% were ASO-positive, with an average ASO value of 296.67±342.50 IU/mL. Throat cultures obtained from four patients all showed negative results. Complete blood count (CBC) tests for 15 patients showed that none of the patients had leukopenia, while 13.3% had leukocytosis, 33.3% had anemia, and 13.3% had thrombocytosis.
ECG and MRI examinations conducted on the patients did not result in any pathological findings, while EEG showed nonspecific epileptic discharges in one patient. Echocardiography detected cardiac rheumatism in 14 of the patients; all 14 had mitral valve regurgitation. The most common type of accompanying cardiac valve involvement was mitral and aortic valve regurgitation (71.42%), followed in descending order by mitral valve regurgitation (63.6%), aortic valve regurgitation (45.5%), tricuspid valve regurgitation (22.7%), pulmonary valve regurgitation (4.5%), and pericardial effusion (4.5%). Left ventricular function and right ventricular function in all 22 patients were normal. Fig. 3 shows the patient distribution based on cardiac valve involvement.
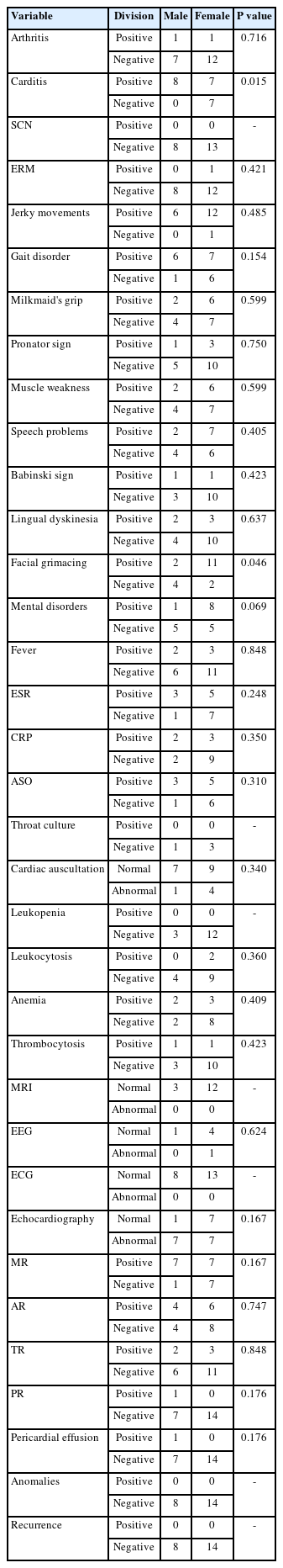
Table 1 lists the relationship between the study variables and the patients' sex. Of eight male patients, all had carditis, while only seven out of 14 female patients had carditis. Carditis was significantly more common in boys than in girls (P<0.05). Furthermore, in patients’ clinical findings, two boys and 11 girls had facial grimacing. Facial grimacing was significantly more common in girls than in boys (P<0.05). There was no meaningful correlation between patients' sex and other variables (P>0.05).
Table 2 shows the relationships between cardiac auscultation and echocardiography findings of the patients. There was no meaningful correlation between the cardiac auscultation and echocardiography results (P>0.05).
Reviewing the statistical indicators of the relationship between hearing a heart murmur and diagnosing carditis on echocardiography, murmur showed a sensitivity of 30.77%, specificity of 87.50%, positive predictive value of 80%, and negative predictive value of 43.75%.
None of the 22 patients had any associated anomalies. In the 1- to 10-year follow-up, none experienced a recurrence of chorea, and one died due to heart problems.
Discussion
The current study aimed to review demographic, clinical, and paraclinical findings on pediatric patients with Sydenham chorea. This study showed that Sydenham chorea can be the only sign of RF and is significant in diagnosing the disease alongside the clinical symptoms. As discussed, the most common symptoms of Sydenham chorea are, in order of diminishing frequency, jerky movements, facial grimacing, gait disorders, mental disorders, speech disorders, muscle weakness, milkmaid's grip, lingual dyskinesia, fever, pronator sign, and Babinski sign. The disease is most common among children between the ages of 7 and 12 years, but may still be seen in other age groups.
Study findings showed more prevalence of Sydenham chorea among girls than among men, and the average age of the patients was 10.09 years. Carditis was more common in men, while the prevalence of facial grimacing was more common in women. However, we believe that, due to the limited number of patient cases, further studies with larger sample sizes are needed in order to be able to interpret the divergent frequencies of carditis and facial grimacing between boys and women.
In 31.8% of patients, chorea was the only sign of RF, while in 68.2%, other major signs were observed, the most common of which was carditis. None of the patients had concurrent arthritis, ERM, or SCN with chorea, which can be due to the fact that major symptoms other than carditis disappear earlier, while carditis and chorea are the two delayed signs. In our study, laboratory tests were positive for ESR, CRP, and ASO in 50%, 31.2%, and 53.3% of the patients, respectively. Throat culture results were negative for all four patients undergoing the test. We believe that throat cultures are usually negative by the time chorea appears, as chorea is a delayed complication of RF.
The most common laboratory findings in patients' CBCs were anemia, leukocytosis, and thrombocytosis. The patients' neuroimaging histories were reviewed, and patients’ brain MRIs were all normal. No correlation was observed between the cardiac auscultation and echocardiography findings. Despite the normal results of cardiac auscultation in patients, echocardiography showed 63.6% of them with rheumatic heart disease. The most common valve involvements were mitral and aortic regurgitations.
Sydenham chorea is a rare sign of acute rheumatic fever (ARF), which is shown by a few patients and can be the only sign. The inflammation process in the CNS involves basal ganglia and caudate nucleus neurons. While the incubation period from the onset of streptococcal pharyngitis until carditis and arthritis symptoms is 3 weeks on average, it is 3 months or longer for Sydenham chorea [1,17]. Most patients with Sydenham chorea are treated and recover within 6 months (typically in 6 weeks); however, there have been cases observed in which the disease persists for 3 years [18]. MRI scans are typically normal, and positron emission tomography scans show elevated levels of metabolism in the striatum. Neurochemical studies have reported reductions in gamma-aminobutyric acid in basal ganglia cells [12,19-23].
There is no specific test to diagnose Sydenham chorea and ARF. The diagnosis of RF is made using the Jones criteria [24]. ARF is diagnosed based on clinical findings and strong clinical suspicion, especially considering that there is no history of acute streptococcal pharyngitis in one-third of the cases [6,10,11].
Chew et al. [25] reviewed the prevalence, epidemiology, and clinical signs of Sydenham chorea and showed an average age of 11.5 years among 37 patients, with most patients (68%) under 12 years old, and a female-to-male ratio of 1.01 to 1. Two-thirds of patients had bilateral chorea, and one-third had unilateral chorea. The most common neurological clinical signs of the patients reported were, in descending frequency, facial grimacing (38%), dysarthria (25%), and gait disorders (21%), with no patients showing signs of seizure. While chorea was the only clinical sign in 65% of the patients, some patients showed other signs of RF as well, including carditis (35%), arthralgia (24%), fever (2%), SCN (11%), arthritis (5%), and ERM (3%) [25].
In a research study conducted by Regmi et al. [26], 16 patients with chorea had carditis, six patients had arthritis, and only four patients had chorea as the only sign of RF. In a study by Ekici et al. [27], the most common clinical signs were behavioral changes and muscle weakness. Tumas et al. [28] reported the most common clinical findings among pediatric patients to be behavioral disorders (40%), dysarthria (39%), and gait disorders (34%). However, in our study, we reported the most common signs to be, in descending order of frequency, jerky movements, facial grimacing, gait disorders, mental disorders, speech problems, muscle weakness, milkmaid's grip, dyskinesia, fever, the pronator sign, and the Babinski sign, with bilateral and unilateral chorea in 78.9% and 21.1% of the patients, respectively. In our study, in 31.8% of the patients, chorea was the only sign of RF, while 68.2% had chorea with other major signs of RF. Two patients had a history of arthritis, and one had a history of ERM. Fifteen patients had carditis, while no SCN was found in any patients.
In the study by Chew et al. [25], EEG showed abnormal findings in four patients, while patients' brain computed tomography scans showed no pathologic findings. In the of Ekici et al. [27], brain MRI showed abnormal nonspecific findings in 47% of the patients with hyper-signal intensities in white matter, brainstem, and caudate nucleus. In our study, no pathological findings were observed in the patients’ brain MRI scans, and EEG also showed nonspecific epileptic discharges in one patient.
In the study by Gurkas et al. [29], recurrence was documented in 16% of patients during 6 months to 9 years of follow-up. In contrast, no patients experienced disease recurrence in the study of Kilic et al. [22] In our study, a 1- to 10-year follow-up showed no recurrence of the disease in the studied patients, of whom only one died due to heart problems.
In conclusion, any child showing symptoms of Sydenham chorea needs to be examined by a physician and, if deemed necessary, undergo further examination or tests. Early treatment would prevent further complications and typically results in full recovery.
This study showed that, among laboratory results, ESR, CRP, and ASO typically show elevated levels in Sydenham chorea patients, which can be helpful for diagnosis. These laboratory results can be employed to diagnose this disease more purposefully despite the lack of any relationship between them and the clinical findings. However, it should be mentioned that laboratory tests such as ESR, CRP, and ASO might be negative, and their negativity might be explained by the interval between the acute bacterial infection and the occurrence of Sydenham’s chorea.
Although holosystolic murmur was only observed in 23.8% of the patients on cardiac auscultation, 63% of the patients showed cardiac rheumatism on echocardiography. These results show the importance of strong clinical suspicion in diagnosing cardiac rheumatism and other cardiac issues by echocardiography.
Further studies are recommended to be conducted at multiple centers and with a higher number of patients in order to obtain a more comprehensive clinical perspective of this disease using statistical methods.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: HMMS. Data curation: HMMS, EH, MBT, and AM. Formal analysis: AP. Methodology: AP. Project administration: HMMS. Visualization: HMMS, MBT, and AM. Writing-original draft: HMMS, EH, and AP. Writing-review & editing: HMMS, EH, and AP.
Acknowledgements
The authors appreciate the cooperation of Mashhad University of Medical Sciences.