Introduction
Infectious meningitis and encephalitis are serious clinical conditions that can have serious and even life-threatening consequences, including hearing loss, cognitive deficits, epilepsy, and death [
1]. Delays in diagnosis and inappropriate treatment are associated with significant morbidity and mortality, especially for bacterial meningitis and herpes simplex virus (HSV) meningoencephalitis [
1,
2]. Additionally, unnecessary antimicrobial treatment and prolonged hospitalization increase antimicrobial resistance and costs [
3,
4]. Although there are many causes of central nervous system (CNS) infections, the signs and symptoms are similar [
5]. To identify the pathogenic agent, lumbar puncture, and cerebrospinal fluid (CSF) collection are needed. CSF analysis may show characteristic findings of a specific etiology, but test results alone cannot accurately identify the causative pathogen. There are heterogeneous diagnostic tests and treatment regimens for meningitis and encephalitis. Conventional testing, including routine analysis, Gram staining, and bacterial culture studies for CSF, can help identify the pathogenic organisms. However, these methods take several days, and their results may be negative despite a suspicion of CNS infection. Clinicians face diagnostic and therapeutic challenges when they encounter invasive CNS infections with a negative CSF Gram stain and culture. Various microbiological tests and molecular biological tests to detect viral and bacterial pathogens and fungal cultures may be necessary, and these tests require a significant volume of CSF.
Rapid diagnosis helps detect invasive and serious infections in the CNS, ensures the early implementation of proper and accurate treatment, and enables the avoidance of unnecessary treatment. In 2015, the BiofireFilmArray Meningitis/Encephalitis (FA ME) panel (BioFire Diagnostics, Salt Lake City, UT, USA) received U.S. Food and Drug Administration approval as a diagnostic tool for CNS infections [
4,
6,
7]. The FA ME panel simultaneously detects 14 pathogens in the CSF, including six bacteria (
Escherichia coli K1,
Haemophilus influenzae,
Listeria monocytogenes,
Neisseria meningitidis,
Streptococcus pneumoniae, and
Streptococcus agalactiae), seven viruses (cytomegalovirus [CMV], enterovirus [EV], HSV-1, HSV-2, human herpesvirus [HHV]-6, human parechovirus [HPeV], varicella zoster virus [VZV]), and one fungus (
Cryptococcus neoformans/gattii). Since the approval of the FA ME panel, many studies have reported its usefulness worldwide, yet only a few studies have evaluated clinical implementation in Asia, including Korea [
8,
9]. In Korea, the FA ME panel was available for only less than 1 year, but it has recently become possible to use this panel again. In this study, we aimed to report our experience with the FA ME panel and evaluate its usefulness for the rapid diagnosis of CNS infectious diseases at a tertiary center in Korea.
Materials and Methods
1. Patients
We retrospectively analyzed the medical records of patients who were suspected of having a CNS infection (fever, headache, vomiting, nuchal rigidity, or other signs of meningeal irritation) and reviewed the results of FA ME panels of the CSF at Pusan National University Hospital between November 2017 and August 2018. The inclusion criteria were the presence of one or more of the following: (1) pleocytosis in the CSF (>5 leukocytes/mm
3 [μL] if the patient was ≥2 months of age or >15 leukocytes/mm
3 [μL] in infants <2 months) [
10]; (2) a positive serological test for a pathogen in serum and/or the CSF; (3) identification of a pathogen in the CSF via multiplex molecular tests; or (4) confirmation of a pathogen by Gram staining and/or culture studies. The exclusion criteria were the presence of one of the following: (1) repeat CSF samples from the same patient; (2) pleocytosis due to identifiable non-infectious causes, such as hemorrhage, tumor, chemicals, and autoimmune reactions; (3) immunocompromised patients; and (4) patients who had a CNS prosthetic material or device. CSF was obtained by lumbar puncture. All specimens were obtained from hospitalized patients or patients who visited the emergency room.
2. Clinical data
Information obtained from medical records included sex, age, comorbidities (e.g., epilepsy in pediatric patients, hypertension, diabetes, heart disease, cerebrovascular disease, respiratory disease, or dementia in adult patients), clinical symptoms at onset of the disease, time intervals from symptom onset to lumbar puncture, antibiotics given within 24 hours prior to lumbar puncture, serological studies, CSF analysis, Gram stain, cultures, FA ME panel results, final diagnosis, and whether the treatment was changed after confirming the results of the panel.
3. Conventional microbiological tests
CSF samples were obtained through lumbar puncture and assessed for cell count, glucose, and protein levels. Bacterial culture and Gram staining of blood and CSF were performed in all patients using standard culture media. Additional molecular biological tests, such as real-time polymerase chain reaction (RT-PCR) and serological tests of the CSF, blood, and stool, were performed in patients suspected of having specific pathogens. The assays were as follows: an RT-PCR assay for EV with the AccuPower Enterovirus RT-PCR Kit (Bioneer, Daejeon, Korea), an RT-PCR assay of blood samples for VZV with the BioCore VZV PCR kit (Biocore, Seoul, Korea), a CMV reagent for blood samples with the Cobas CMV reagent (Roche, Basel, Switzerland), an RT-PCR assay of blood samples for Epstein-Barr virus (EBV) with the EBV ELITe MGB kit (ELITechGroup, Puteaux, France), and an RT-PCR assay of CSF samples for Mycobacterium tuberculosis with the AdvanSure TB/NTM PCR kit (LG Life Sciences, Seoul, Korea). RT-PCR testing of the CSF for HPeV was not available at our center.
4. The FA ME panel test
CSF samples were obtained from subjects through lumbar puncture, and the FA ME panel test was performed according to the manufacturer’s instructions (FA ME PCR panel). CSF samples were combined with the FilmArray Sample Buffer at a 1:4 ratio and injected into an FA pouch. The FA ME panel test consists of automated nucleic acid extraction, reverse transcription, and nucleic acid amplification. The FA ME panel requires 200 μL of CSF and simultaneously tests for six bacteria (
E. coli K1,
H. influenzae,
L. monocytogenes,
N. meningitidis,
S. pneumoniae, and
S. agalactiae), seven viruses (CMV, EV, HSV-1 and -2, HHV-6, HPeV, and VZV), and one fungus (
C. neoformans/gattii). The FA ME panel test was performed during the opening hours of the microbiology laboratory from 9:00 AM to 4:00 PM on weekdays. The FA ME turnaround time was defined as the time from the arrival of CSF to the laboratory to the report of the identified organisms. The time only included the period when the specimen stayed for testing in the laboratory and excluded the time the laboratory was closed on weekends. All CSF specimens were stored either refrigerated (4°C) or frozen (-70°C) safely and freshly until testing with the FA ME panel could be completed [
11].
5. Data and discordant results analysis
The results of the FA ME panel were compared with the results of conventional tests to identify pathogens. Results were considered discordant when the results of the FA ME panel did not agree with those of the conventional tests for the specific target pathogens represented on the multiplex panel [
12].
6. Statistical analysis
Statistical analyses were performed using SPSS version 27.0 (IBM Corp, Armonk, NY, USA). For comparisons of categorical data (pathogens of positive results, clinical symptoms), the two-tailed chi-square test or the Fisher exact test was performed. The independent-sample t-test was performed for continuous variables with a normal distribution (age, time from symptom to test, turnaround time, laboratory findings, and CSF findings). The Mann-Whitney test was used for non-normally distributed continuous variables. Multivariate logistic regression was used to identify factors that were independently associated with positivity of the FA ME panel. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to test differences within groups. Receiver operating characteristic curves were generated and the area under the curve was calculated to confirm the cut-off points and calculate the sensitivity and specificity. In all analyses, P<0.05 was considered to indicate statistical significance.
7. Standard protocol approval
This study was approved by the Institutional Review Board of Pusan National University Hospital (1812-017-074). All studies followed the tenets of the Declaration of Helsinki, and written informed consent was obtained from the parents or legal guardians of all patients.
Discussion
A rapid and accurate diagnosis of suspected CNS infections is critical given the associated risks of prolonged antibacterial exposure, diagnostic challenges, long-term hospitalization, costs, untreated CNS infections, and significant morbidity and mortality [
6,
7]. Conventional tests, such as CSF cultures, are considered the gold standard but often require more than 48 to 72 hours to produce results. In addition, conventional methods’ accuracy may be affected by antibiotic administration prior to the testing. Routine testing may produce false positives or negatives due to insufficient number of samples or incorrect sample handling [
7,
8]. These limitations of conventional tests could be overcome by the FA ME panel technology. Fast, comprehensive, and sensitive multiplexed PCR for common pathogenic causes of meningitis and encephalitis is a useful addition to current strategies and has the potential to allow the safe withdrawal of antibacterial coverage. Previous studies have shown the potential benefits of this technique in identifying treatable pathogens and estimated that the median diagnosis time from collection to outcome was approximately 3 hours [
9,
13,
14].
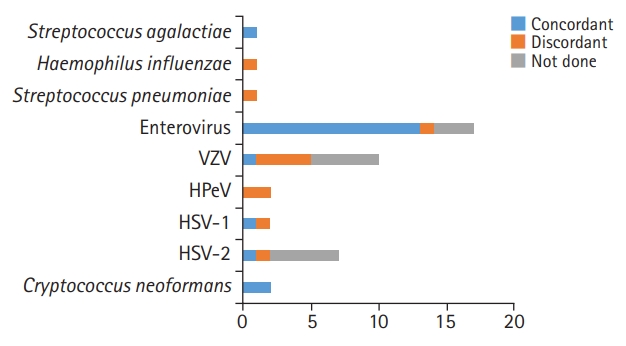
In this study, we investigated the performance of the FA ME panel for the detection of bacterial, viral, or fungal pathogens in the CSF at a tertiary center. Overall, the panel test had a concordance rate of 33.3% (1/3) for bacteria, 94.1% (16/17) for viruses, and 100% (2/2) for fungi in patients who underwent both the FA ME panel and conventional tests. Viruses were the main pathogens identified, in agreement with previous studies showing that 57% more frequently, 60% to 80% of detected pathogens were viruses [
5,
6,
8,
15]. In our study, there were some discordant results (
Fig. 1). Ten out of 43 positive FA ME panel samples (23.3%) had negative results in conventional studies, similar to the results of previous studies [
6]. If antibiotics were administered before the lumbar puncture, the CSF culture test may be negative. In this study, a patient with a positive result for
S. pneumoniae was given antibiotics prior to the test. Patients who were positive for VZV, HSV-1, and HSV-2 started acyclovir regarding the panel results. The other patients with positive results for HPeV and EV had relatively fair general conditions, so they continued to receive conservative treatment. Three patients with negative FA ME results had positive results on conventional tests for EV, HSV-1, and EBV, respectively. Viral loads close to the detection limit have been shown to account for some of these false-negative results [
5]. The exact cause of the discordant results cannot be explained, but some may have been caused by contamination of the samples during the test configuration or the test process. Regarding potential contamination, clinical and laboratory measures have been proposed to decrease discordant outcomes [
16]. CSF sample collection and handling should avoid microbial contamination, which can lead to false results that may delay an accurate diagnosis. In addition, a diagnosis of CNS infection depends not only on laboratory tests, such as CSF culture, but also on patients’ clinical symptoms, age, and comorbidities. For example, especially very early in the illness, a test can be normal despite a CNS infection. In patients who have received empirical antimicrobial treatment before the first lumbar puncture, the CSF findings will probably be modified (e.g., negative results). Therefore, more clinical experience will be needed to further assess the FA ME panel, and clinicians also need to understand its limitations. A treatment direction must be established by considering clinical information and results of other laboratory tests, including conventional tests.
We investigated clinical data that could be related to FA ME panel results. In a comparison of the positive and negative FA ME panel groups (
Table 3), there were several factors (pediatric or adult status, age, CSF lymphocyte count, fever, visual disturbance, conservative treatment, intravenous antibiotics, acyclovir, steroid, IVIG, aseptic meningitis) that showed remarkable differences between the positive and negative groups. Among these factors, age group (i.e., pediatric patients) and acyclovir use were significantly related to positive results according to the multiple logistic regression analysis (
Table 4). First, we suggest that the FA ME panel can have a comparable diagnostic yield and more rapid time to diagnosis than conventional clinical testing, especially in pediatric patients with suspected CNS infections. We believe that the FA ME panel has considerable potential to improve clinical outcomes through earlier optimization of antimicrobial regimens. For example, in this study, 38 CSF samples were positive for viral pathogens. EV (17 cases, 44.7%) was the most common virus detected in our cohort, particularly among pediatric patients (14 cases, 82.4%). With routine diagnostic tests such as serum tests, physicians could not reliably distinguish between children with viral infections and those with bacterial infections, especially in infants, who have non-specific symptoms such as fever or changes in behavior, which make the diagnosis difficult [
6]. Many studies have shown that EV detection in children with fever can rule out the accompanying risk of bacterial infection early [
17,
18] and EV-positive children can be treated efficiently without unnecessary antibiotic treatment [
17]. In addition, previous studies have shown that the fast detection of EV can reduce unnecessary antibiotic treatment, hospitalization, and hospital expenses [
13,
18-
21]. We also confirmed that rapid EV detection and the short turnaround time of the FA ME panel test can be especially beneficial in pediatric patients. Clinically, management decisions for infants who are positive for EV can suggest how the treatment process can change in the future following rapid diagnosis. Second, of the 14 pathogens that FA ME panel can detect, three viruses (VZV, HSV-1, and HSV-2) need acyclovir for treatment [
22]. Acyclovir was administered in 44.2% (19/43) of the panel-positive patients in our study. We believe that more rapid viral pathogen detection by the FA ME panel has the potential to enable the more judicious use of antimicrobial and antiviral agents, particularly acyclovir.
Early diagnosis followed by appropriate medical management can have a favorable effect on outcomes. Whenever a physician suspects meningitis, a lumbar puncture should be undertaken. Especially in neonates, the procedure should be considered when sepsis is suspected, because meningitis accompanies sepsis in 20% to 25% of cases [
18]. The FA ME panel, as a rapid diagnostic platform, can affect the progression of treatment and final diagnosis. In our study, 32 (74.4%) patients’ treatment changed after the FA ME panel results were confirmed to be positive. New antibiotics or acyclovir was started in some cases, and the previous treatment was also discontinued. Half of them (n=16) were pediatric patients. Antibiotics were discontinued in eight of the 24 panel-positive pediatric patients after the panel results were confirmed. Two of these patients had HPeV, and four had EV; all of these patients were less than 100 days old. They were brought to the hospital for fever and began empirical antibiotics with initially suspected sepsis. Their general condition was fair and there were no specific abnormal findings on other tests; hence, antibiotic treatment was stopped and conservative treatment was maintained for symptom control after the panel results were confirmed. In two cases with confirmed VZV and HSV-2 by the FA ME panel test, antibiotics were stopped, and acyclovir was administered. Of 19 adult patients who were positive on the FA ME panel test, antibiotics were discontinued in three after panel test confirmation, and one received acyclovir for HSV-2. The two patients who were confirmed to have fungal infections stopped taking antibiotics and received anti-fungal treatments. Antibiotics could be discontinued in all these patients because their vital signs were stable, and there were no findings suggesting bacterial infection in physical or neurological examinations and laboratory tests. In other words, the treatment choice does not depend only on the results of the FA ME panel or conventional tests. Instead, the treatment was decided based on a thorough consideration of both the FA ME panel results and other factors (cloudy CSF, increased white blood cell count in the CSF, predominance of polymorphonuclear leukocytes, a low glucose concentration in relation to serum value, a raised concentration of protein, vital signs, and clinical symptoms).
Our study had some limitations. First, this study investigated the use of the FA ME panel test for CNS pathogens in both adults and pediatric patients, but the number of positive cases was limited, and the sample size was too small for statistical comparisons. Second, since this was a retrospective study of patients treated at a single tertiary hospital, the consistency of data collection could have potentially been limited, although most data were easily retrieved. We limited the use of samples that had already been identified as positive for our study. Third, the CSF of some of our study samples was not subjected to conventional testing, which made it difficult to accurately measure the concordance rate of the entire patient cohort. Although we tried to further analyze all discordant results, we were unable to resolve the discrepancies due to insufficient amounts of CSF, the lack of available verification tests, or limited access to patient or laboratory records. Fourth, for E. coli, L. monocytogenes, N. meningitidis, CMV, and HHV-6, no positive samples were identified; thus, our study had a limited ability to evaluate the performance of the FA ME panel for those pathogens. Finally, our study was conducted only in a short period of approximately 11 months because the FA ME panel test was only available during that period in Korea. The FA ME panel, which diagnoses the cause of meningitis and encephalitis within an hour, is on the verge of disappearing after entering the reimbursement system, because the reimbursement fee for the FA ME test is not close to its cost.
We compared the performance of standard diagnostic tools to the FA ME panel in 129 patients. The FA ME panel cannot test for all causative organisms of CNS infections, but is a useful way to quickly identify a pathogen. Pediatric patients are often diagnosed with sepsis, and lumbar puncture is frequently performed. The FA ME panel can quickly detect the most common pathogens that cause meningitis and encephalitis in infants and play an important role in diagnosis, thereby having the potential to reduce the use of unnecessary antibiotics, shorten hospitalization, and improve the prognosis.