Two Cases of Norovirus Gastroenteritis Associated with Severe Neurologic Complications
Article information
Norovirus, along with rotavirus, is a common virus that causes gastroenteritis in children [1]. Complications related to norovirus enteritis are rare, and the prognosis is good. However, various clinical features and poor prognoses have been reported in norovirus-associated encephalopathy [2,3]. Herein, we report two pediatric cases of norovirus infection with severe neurologic complications.
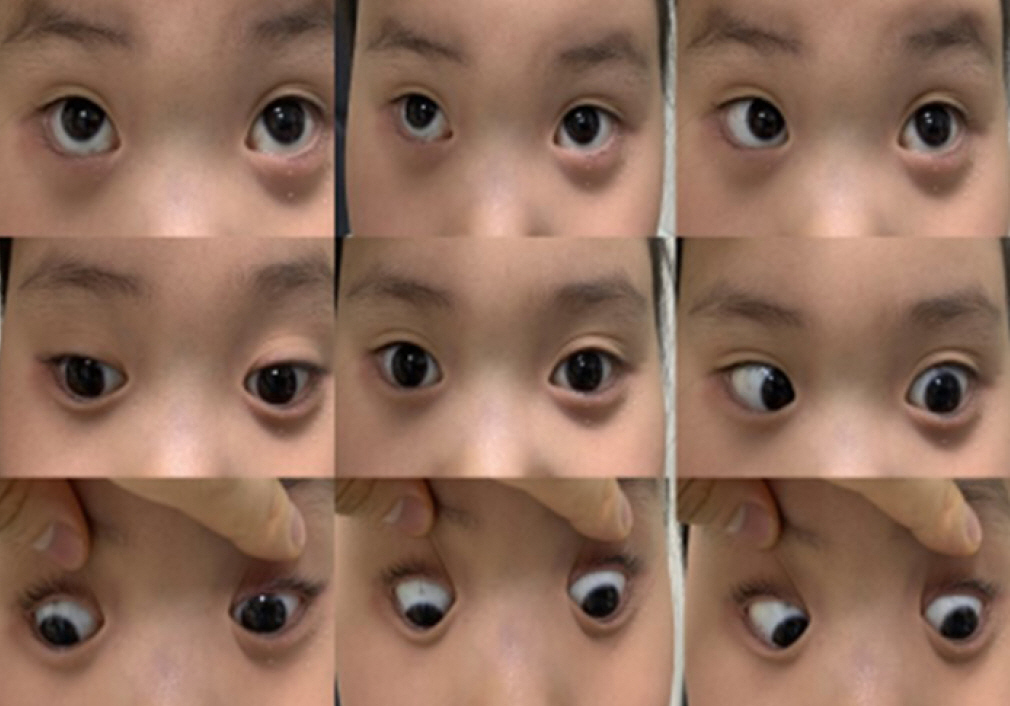
A 7-year-old girl (patient A) visited the emergency room (ER) of a local hospital for diplopia, impaired vision, eye movement disorder, and mild neck stiffness that had lasted for 5 days. Days before developing ocular symptoms, she had abdominal pain, was diagnosed with enteritis, and took medications to alleviate the symptoms. Her mental status was alert, and the Glasgow Coma Scale score was 15. In a workup at a local hospital, brain magnetic resonance imaging (MRI) and angiography showed papilledema. She received mannitol for papilledema and intravenous methylprednisolone (1 mg/kg/day) for 4 days for sixth cranial nerve palsy, followed by dexamethasone (0.5 mg/kg/day) for the next 6 days. During hospitalization, transient ataxia and bilateral periorbital pain were noted. Starting on hospitalization day 9, her symptoms improved, and a dexamethasone dose of 0.25 mg/kg/day was administered for 2 days. Subsequently, a reduced dexamethasone dose (0.125 mg/kg/day) was administered for the next 2 days. She was discharged on hospitalization day 13; the dexamethasone dose was tapered for 4 days and discontinued. However, on day 4 after discharge, her symptoms worsened again. Hence, she was transferred to our hospital.
On transfer to our hospital, she had fixed dilated pupils, and her left eye had visual acuity of light perception and exhibited left gaze palsy (Fig. 1). Her right eye had a visual acuity of 0.04. On fundoscopy, stage 4 papilledema was confirmed in both eyes, and a slight decrease in the deep tendon reflex was observed. Whole-spine MRI and an autoantibody panel (anti-aquaporin 4 antibody [AQP4], anti-neurofilament antibody [NF], and anti-myelin oligodendrocyte glycoprotein [MOG]) revealed normal findings. Stool virus polymerase chain reaction (PCR) findings showed positivity for norovirus genogroup II (GII). She received intravenous immunoglobulin (IVIG) at a dose of 0.4 g/kg for 5 days and methylprednisolone (30 mg/kg/day) for 3 days. Her abdominal symptoms and visual acuity in the right eye improved. Further improvement was noted after acetazolamide was administered.
A previously healthy 8-month-old girl (patient B) was brought to our ER with an ongoing seizure for 20 minutes. The patient had been treated for otitis media 2 weeks ago and had been vomiting for 2 days. Her temperature was 40°C. She was treated with lorazepam, phenytoin, phenobarbital, and midazolam. She was admitted to the intensive care unit, where diarrhea was noted. Her laboratory findings were as follows: hemoglobin, 12.3 g/dL; leukocyte count, 13.37×106/L; C-reactive protein, 0.18 mg/dL; glucose, 308 mg/dL; ammonium, 108 IU/L; and serum sodium, 150 mEq/L. Venous blood gases showed metabolic acidosis. PCR analysis of a respiratory sample from the patient showed negative results for norovirus GII, but that of the stool sample showed positive results for norovirus GII. Empiric treatment was initiated with vancomycin and acyclovir, and IVIG (0.5 g/kg/day for 3 days) was administered for encephalitis. Hydrocortisone and dopamine were administered for hypotensive shock. On hospitalization day 2, the coagulation studies were compatible with disseminated intravascular coagulopathy (DIC). Brain MRI performed on hospitalization day 2 demonstrated a high signal intensity along the cerebral hemisphere sulci with enhancement on diffuse fluid-attenuated inversion recovery imaging and diffusion restriction in the posterior parietal and occipital cortex and subcortex in diffusion-weighted images, indicative of meningoencephalitis and post-ictal changes (Fig. 2). She experienced further myoclonic seizures; therefore, midazolam and levetiracetam were added. A continuous encephalogram showed suppressed background activities and continuous right central spike discharges at 3- to 5-second intervals. Despite receiving aggressive drug administration, the cerebral edema worsened and DIC did not improve, resulting in death due to multiorgan failure.
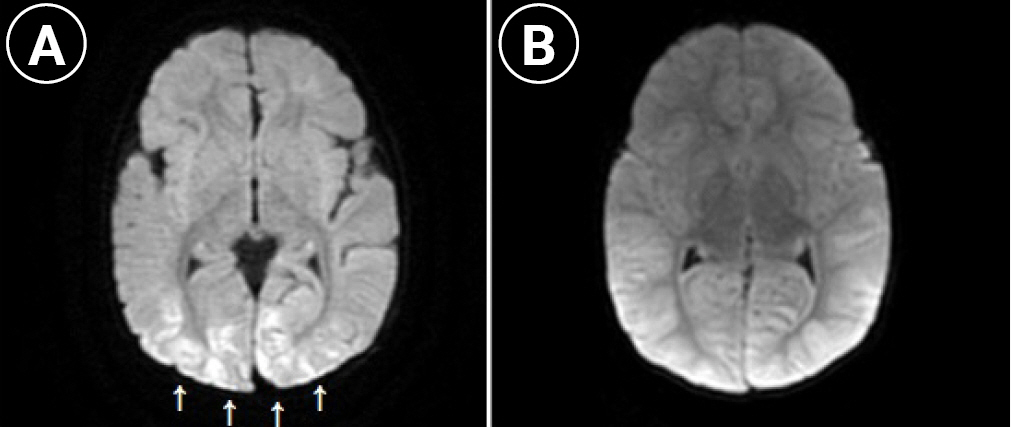
Axial diffusion weighted brain magnetic resonance imaging of the patient B (A: 2nd day; B: 5th day of hospitalization). (A) Depicting diffusely diffusion high signal intensity lesions (white arrows) predominantly occipital lobe on diffusion weighted image, (B) diffuse cortical swelling in cortex and subcortex of both cerebral hemisphere with tonsillar and transtentorial.
Although there have been few reports of norovirus-associated encephalopathy, most have a benign neurological prognosis [4]. However, in these cases, serious complications were identified. Norovirus was detected in the patients’ stool samples by multiplex reverse-transcription PCR. Although no cerebrospinal fluid (CSF) study was conducted, no other causes of encephalopathy were found. Norovirus remained the strongest candidate in the differential diagnosis.
In the case of patient A, the possibility that a previous norovirus infection appeared in the form of Miller Fisher syndrome (MFS) cannot be excluded. In a previous case report, MFS was probably attributed to norovirus infection [5]. There was no eye pain or ptosis, and the patient had decreased visual acuity due to papillary edema, diplopia, and lateral palsy. Therefore, it was considered a sixth nerve abnormality rather than a second or third cranial nerve abnormality. Neuromyelitis optica was also excluded because the spine MRI was normal.
A CSF study could not be conducted in patient B. As her vital signs, including blood pressure, were unstable, this examination was scheduled for later. Other viruses, such as herpes simplex virus (HSV) and enterovirus, which cause encephalitis, were also considered. However, lesions of the limbic system found in most patients with HSV encephalitis were not observed on brain MRI, and there were no other history or physical examination findings suggestive of enterovirus infection. Similar brain MRI findings to those of patient B were also found in a previously healthy 15-month-old girl with norovirus-associated encephalopathy [6].
The utility of CSF studies to diagnose norovirus-associated encephalopathy has not been established. The norovirus genome was detected in the CSF sample of a 23-month-old girl with altered consciousness [2]. Whether norovirus replicates in the central nervous system remains unclear.
Studies have suggested that cytokines in the serum and CSF play a role in acute encephalopathy [7]. A report demonstrated increased interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) levels in the CSF of patients with influenza, suggesting that cytokine levels are associated with the sequelae and prognosis [8].
A study on the cytokine profiles in the CSF of patients with norovirus reported markedly elevated IL-6, IL-10, interferon-γ, and TNF-α levels [6]. Cytokines, such as IL-6, predict disease severity and prognosis in viral encephalitis [9].
The encephalopathy in our cases could have been related to increased cytokine levels rather than direct viral invasion. The immune response to norovirus is not yet fully understood. We suggest that increased cytokine levels in the CSF may lead to severe encephalopathy. However, further studies are warranted to confirm this possibility.
This study was approved by the Research Ethics Committee of Inha University Hospital (approval number: 2022-04-005). The requirement for written informed consent was waived because of the retrospective nature of the study.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: MJY and YSK. Data curation: MJY, DJH, DHK, and YSK. Formal analysis: YSK. Methodology: MJY and YSK. Project administration: MJY and YSK. Visualization: MJY, DJH, and YSK. Writing-original draft: MJY. Writing-review & editing: MJY, DHK, and YSK.