Oral Loading of Phenobarbital to Achieve Therapeutic Effects in Pediatric Patients with Acute Repetitive Seizures
Article information
Abstract
Purpose
Intravenous phenobarbital has not been available at our institution since 2019. This study aimed to determine the effectiveness of oral phenobarbital loading at a dose of 15 to 20 mg/kg to achieve therapeutic serum levels and clinical seizure control in pediatric patients with acute repetitive seizures.
Methods
This is a retrospective single-center review of the medical records of pediatric patients admitted for acute repetitive seizures (from January 2019 to June 2022) at the Philippine Children’s Medical Center who were given an oral phenobarbital loading dose of 15 to 20 mg/kg with serum level measurements taken within 48 hours after oral loading.
Results
Eleven patients were given a single oral phenobarbital loading dose of 15 to 20 mg/kg, while 14 were given two doses of 10 mg/kg 12 hours apart. All 25 patients achieved therapeutic serum levels within 48 hours post-loading. Higher serum levels were seen at 48 hours (median, 19.8 μg/mL at 24 hours vs. 24.4 μg/mL at 48 hours). This difference was statistically significant at 5% (P=0.023), and the majority achieved adequate seizure control without requiring additional anti-seizure medications (76%). Transient drowsiness was the most commonly documented adverse effect.
Conclusion
Oral loading of phenobarbital at a dose of 15 to 20 mg/kg given as a single dose or in two divided doses is an effective and safe alternative to achieve therapeutic serum levels and adequate clinical seizure control at 24 hours post-loading. This may be a promising and useful intervention at centers without available intravenous phenobarbital.
Introduction
One of the oldest antiseizure medications (ASMs), phenobarbital is a gamma-aminobutyric acid (GABA) inhibitor that decreases neuronal excitability by increasing the frequency of chloride channel openings. It has almost complete (95%) oral bioavailability, low total clearance, and a long half-life of 72 to 144 hours [1-3]. Phenobarbital is effective for a wide range of seizure types. Its efficacy in managing status epilepticus and acute seizures is well-established at a dose of 15 to 20 mg/kg in children to achieve rapid plasma levels in a short period of time [2-6]. In resource-poor countries, including the Philippines, phenobarbital is still the most frequent first-line long-acting ASM and is more cost-efficient than newer ASMs. However, since 2019, intravenous phenobarbital has been unavailable at our institution, while the oral form has remained accessible. Although the maximum probability of its efficacy is delayed by up to 12 hours when given via the oral route, a few studies have utilized oral phenobarbital loading. In the 1980s, children at high risk for seizure relapse were given an oral phenobarbital dose of 15 mg/kg followed by a second dose of 3 to 5 mg/kg 12 hours later. Monitoring serum phenobarbital levels showed that the effective therapeutic range (12.6 to 22.3 mg/L) was achieved as early as 1-hour post-loading, with no seizure recurrences observed [6,7]. Oral phenobarbital was also administered as an alternative regimen to pediatric patients with refractory status epilepticus in South Africa as a single dose of 20 mg/kg. Serum concentrations at different time points post-loading demonstrated therapeutic serum levels as early as 4 hours, making this method effective even in an acute setting [8]. Neonates who presented with seizures were also given oral phenobarbital as a single dose of 20 mg/kg with serial serum level measurements taken. Therapeutic levels were seen in as early as 0.5 hours, and at 12 hours post-loading, 80% of the neonates had adequate serum levels [9]. The common acute adverse effects of phenobarbital include sedation, respiratory distress, and liver injuries, but these previous studies demonstrated that children could tolerate enteral loading of phenobarbital to a maximum dose of 80 mg/kg with no evidence of hypotension, arrhythmia, respiratory depression, or deterioration in liver function [6-9].
In our setting, oral phenobarbital has been used as a second- or third-line long-acting ASM for seizure control in patients with repetitive seizures at a total dose of 15 to 20 mg/kg, given as a single dose or in staggered doses to be completed in 24 to 48 hours [10]. As of this writing, only a few studies are available for review, and no local data are available. This study thus aimed to determine whether oral phenobarbital can produce adequate serum therapeutic levels within 24 to 48 hours when given either as a single dose in a range of 15 to 20 mg/kg or in staggered doses of 10 mg/kg 12 hours apart. Specifically, this study also compared the different oral dosing regimens of phenobarbital in terms of the proportion of patients who achieved therapeutic serum levels, the rate of clinical seizure recurrence, and the acute adverse effects that were documented.
Materials and Methods
1. Patient selection
A retrospective review was conducted of the medical records of pediatric inpatients admitted from January 2019 to June 2022 for acute repetitive seizures at the Philippine Children’s Medical Center. Acute repetitive seizures previously known as cluster seizures are defined most commonly as seizures occuring more than three times in 24 hours with interictal period of 8 hours or less, or seizures occuring more than two times in 6 to less than 48 hours [11]. Patients aged 1 month to 18 years old were included if they were given either a single oral loading dose of 15 to 20 mg/kg/dose or two doses of 10 mg/kg 12 hours apart, with a serum level measurement taken within 24 to 48 hours from the initial loading dose. These patients may have had newly diagnosed epilepsy or were known epilepsy patients already on maintenance ASMs. Patients who were already maintained on phenobarbital were included if they had a history of missed doses for more than 24 hours or had laboratory-confirmed subtherapeutic levels. Patients who were given oral phenobarbital after another first-line ASM failed to control seizures were also included. Fig. 1 shows the distribution of the data samples collected.
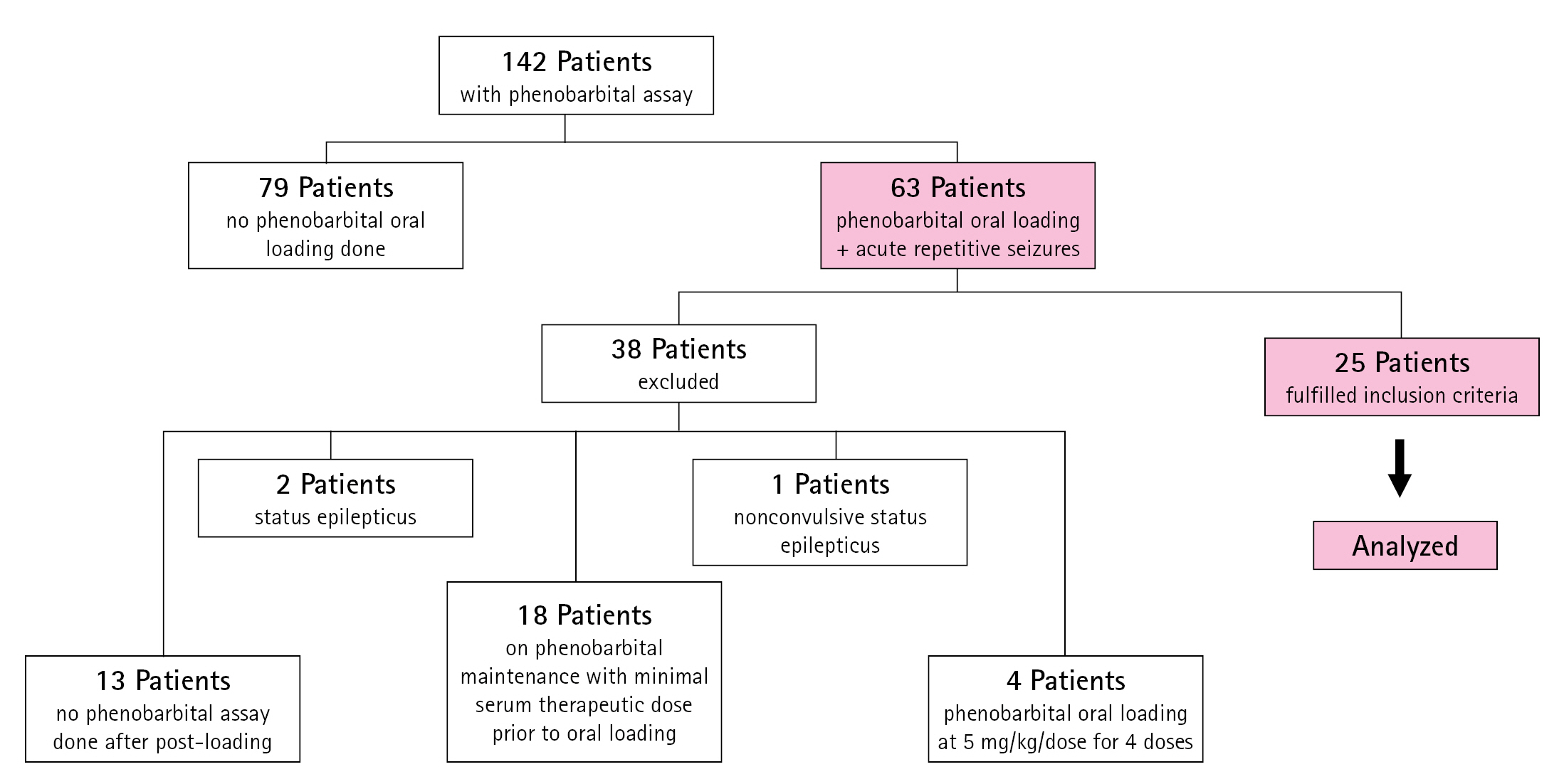
Distribution of data samples included in the study. Within the study period, 142 patients had medical records documenting a phenobarbital assay. Only 63 patients had acute repetitive seizures and oral loading of phenobarbital. Thirty-eight patients did not meet the inclusion criteria and were excluded for the following reasons: 13 patients had no phenobarbital serum level measurements taken after the loading dose, 18 underwent oral loading but were already on phenobarbital maintenance with minimum therapeutic serum levels; four were given phenobarbital oral loading at 5 mg/kg/dose for 4 doses over 48 hours; two were given an oral phenobarbital loading dose to control clinical status epilepticus, and one had nonconvulsive status epilepticus.
Only convenience sampling was possible—The initial sample size computation was a minimum of 20 patients per arm satisfying the inclusion criteria, to determine, at 80% power, the effect of oral phenobarbital loading on serum phenobarbital levels, assuming that each subject had three measurements taken and intersubject variability was 50%. However, the number of samples obtained was not achieved from the total charts reviewed, and a total sample size of only 25 was obtained in this study. A total of 142 inpatient medical records with phenobarbital assays from the study period were reviewed. Of these, only 63 patients were given oral phenobarbital loading for acute repetitive seizures. Thirty-eight patients were excluded for various reasons (Fig. 1). The 25 patients who met the inclusion criteria were categorized into two groups: those who received a single dose and those with two doses. Seizure recurrences after oral loading and additional doses of phenobarbital or ASMs given to achieve clinical seizure control were also documented. If available from the records, information on liver function tests conducted within 24 to 48 hours after oral loading was also collected.
2. Statistical analysis
Summary statistics were reported as median (interquartile range [IQR]) for quantitative variables with skewed distribution and as percentage (count) for qualitative measures. The minimum and maximum values of continuous data were also reported. The Shapiro-Wilk test was used to determine whether continuous variables deviated from a Gaussian (normal) distribution. The chi-square test of independence was used to determine the significance of associations between two qualitative variables. The Mann Whitney U test was used to compare the serum phenobarbital levels between two groups. Statistical significance was based on a P≤0.05. Stata version 15 (Stata Corp LLC, College Station, TX, USA) was used for data processing and analysis.
3. Ethics
Ethical approval was obtained from the Philippine Children's Medical center Institutional Review Board (IR-EC #: PRCMC IR-EC 2021-041). This study adhered to the ethical consideration and principles set out in the Data Privacy Act of 2021 and the National Ethical Guidelines for Health and Health-Related research of 2017. Written informed consent from the patients was waived due to the retrospective nature of the study and patients' information and results gathered were strictly kept confidential by removing any identifiers such as names, addresses, and contact numbers before further usage and analysis. The investigator, co-investigator, and supervising investigators have completed the Good Clinical Practice training on the responsible conduct of research with human subjects.
Results
1. Demographic and clinical data
Eleven patients (45.5% male) were given a single dose of oral phenobarbital loading, while 14 patients (50% male) were given two divided doses (Table 1). The median age was 2.9 years. Sixteen patients (64%) were diagnosed with epilepsy with structural etiologies. Focal motor seizures were seen in 23 patients (92%), and a co-existing illness during seizure occurrence was noted in 17 patients (68%), with pneumonia being the most common (10 patients, 40%). Five patients (20%) were already on phenobarbital maintenance, and three had missed doses for more than 24 hours for various reasons. Seven patients (28%) were maintained on a different ASM, with oral levetiracetam being the most common (in eight patients), followed by oral phenytoin. An intravenous benzodiazepine (diazepam/midazolam) was administered to 14 patients (56%) prior to oral phenobarbital loading. Intravenous levetiracetam was started in 10 patients (40%), followed by intravenous valproic acid in three patients (12%) without adequate seizure control before being given oral phenobarbital loading.
2. Determination of serum phenobarbital levels
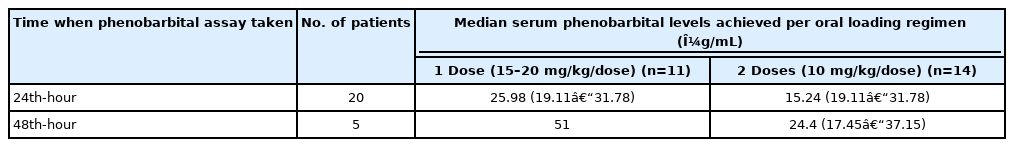
Serum phenobarbital levels were determined once within 24 to 48 hours after oral loading (Fig. 2). The therapeutic range was defined as a serum level of 10 to 40 µg/mL—As indicated in our institution’s laboratory values. All 25 patients were able to achieve therapeutic serum levels within 48 hours post-loading. Twenty patients had an assay taken within 24 hours post-loading, with a median value of 19.8 μg/mL (IQR, 15.05 to 26.05), range from 13.5 to 38.5 μg/mL. These patients were 10 of 11 (90.9%) with single dose and 10 of 14 (71.4%) with two doses. There was a significant difference on the median serum levels between one dose group (25.98 mg/mL; IQR, 19.11 to 31.78) and two dose group (15.24 mg/mL; IQR, 13.96 to 27.37).
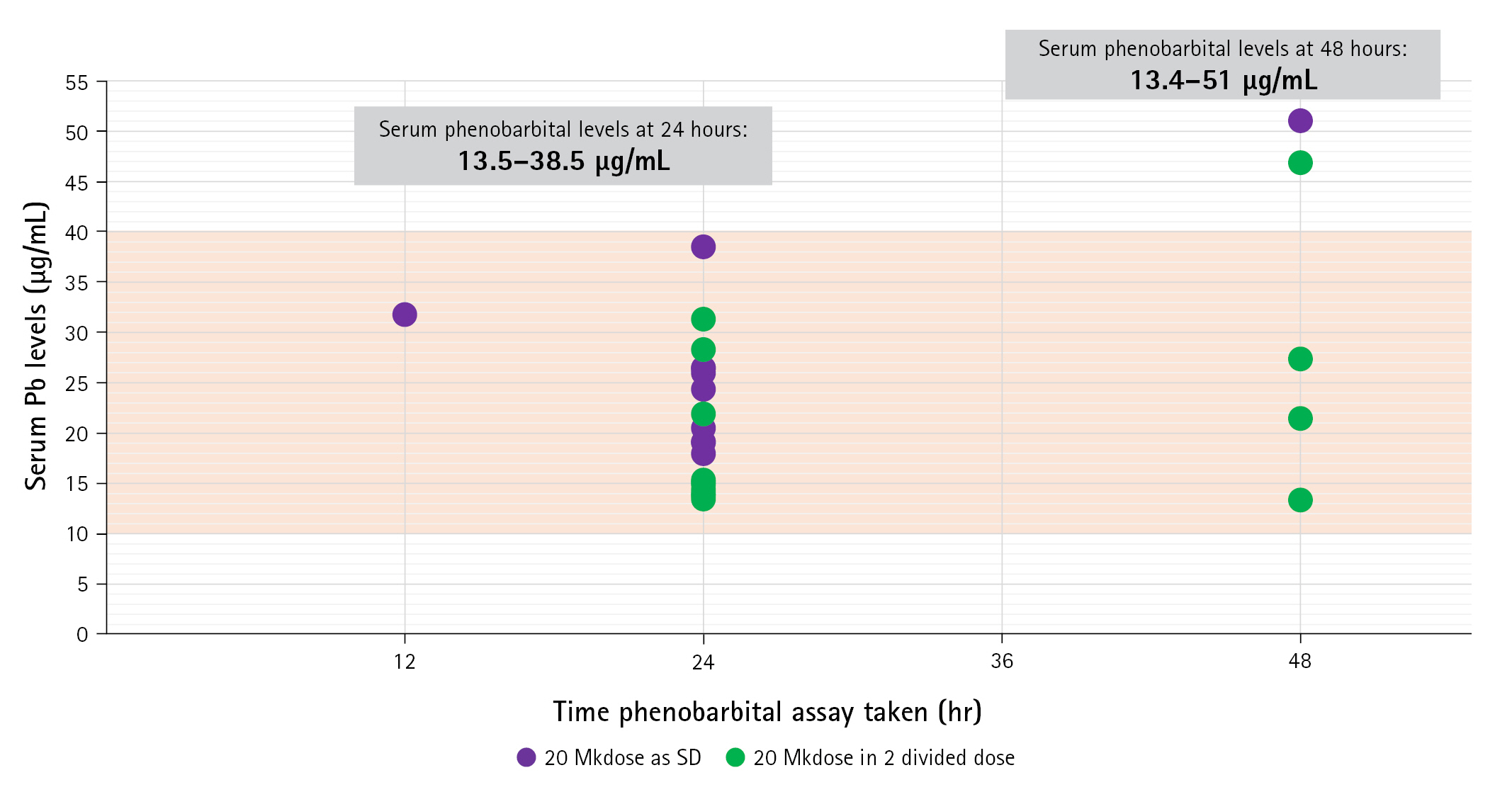
Serum phenobarbital levels attained within 24 to 48 hours post-loading. The X-axis corresponds to the time in hours when the measurement was made, while the Y-axis corresponds to the serum levels of each patient. Patients who received a single dose of phenobarbital oral loading are shown in purple, while those who received two divided doses are colored green. The therapeutic range is marked by the orange area. Serum values ranged from 13.5 to 38.5 μg/mL at 24 hours and from 13.4 to 51 μg/mL at 48 hours.
Five patients had an assay determined on the 48th hour post-loading with median serum phenobarbital levels of 27.4 μg/mL (IQR, 17.2 to 48.9), range from 13.4 to 51.0 μg/mL. Four of these received two doses (4 of 14, 28.6%) and one had a single dose (1 of 11, 9.1%). The median serum level in the four patients with two doses was 24.4 μg/mL (IQR, 17.45 to 37.15), range from 13.4 to 46.9 μg/mL.
The distribution of these patients and comparison between the two regimens did not show any statistical difference (P=0.245) in achieving therapeutic levels (Table 3). At 24 hours, higher median serum levels were found in patients given the single-dose regimen than in those given two doses (P=0.023) (Table 2).

Distribution and comparison of patients who achieved serum therapeutic levels after oral loading of phenobarbital
The overall median serum phenobarbital level was 21.5 mg/mL (IQR, 15.14 to 27.37), range from 13.4 to 51.0 mg/mL. The median serum levels within 24 hours and 24 to 48 hours were 19.8 mg/mL (IQR, 15.02 to 26.45) and 27.4 mg/mL (IQR, 21.46 to 46.86). A comparison between these two groups showed marginal results (P=0.055), suggesting that the actual difference in serum levels could be either significant or not. This warrants further investigation through similar studies on a larger scale.
3. Seizure control and adverse effects
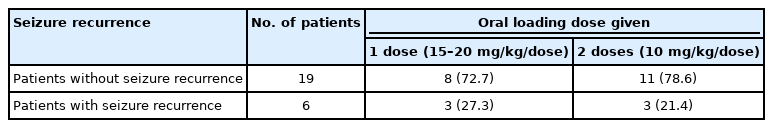
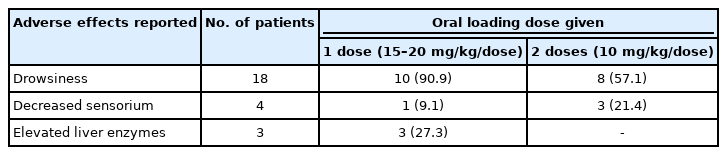
Clinical to seizure control between the two groups was not significantly different (P=0.548) (Table 4). Drowsiness was recorded in 18 patients (75%), without a significant difference between the two groups (P≥0.067) (Table 5).
Discussion
Acute repetitive seizures are neurologic emergencies that may progress to status epilepticus if left untreated. Intravenous phenobarbital is a well-established second-line ASM used for acute repetitive seizures when the recommended benzodiazepines fail to control seizures [11-15]. In this retrospective review, children with acute repetitive seizures were given oral phenobarbital instead of the intravenous form due to the latter’s unavailability. As there are still no set recommendations for the actual doses and use of oral phenobarbital loading at our institution, this method was based on the available related literature and the attending neurologists' experience.
Acceptable serum therapeutic levels may vary across laboratories, but are considered effective at 10 to 40 μg/mL [10]. After oral loading via a single dose or in two divided doses, the patients in this study were able to achieve therapeutic serum levels as early as 12 hours, with higher serum values at the 24-hour determination. Comparing these two dosing regimens demonstrated that higher serum levels were achieved at 24 hours with the single-dose regimen than with the two-dose regimen. In young children and infants, the bioavailability of phenobarbital is less than 50%, with systemic clearance of as long as 70 hours. A higher gastric pH in children also causes a decrease in the absorption rate [16,17]. These factors may contribute to the higher levels documented using the single dose regimen in the 24-hour test. At 48 hours, higher serum levels were seen, possibly due to the additional maintenance doses administered a few hours before the determination of serum concentration levels. These results show that an initial high oral loading dose is adequate to provide rapid plasma levels, which can be maintained with additional doses over the following days.
Phenobarbital is effective in focal seizures, the most common seizure type seen in this study [18]. Most patients who were given oral phenobarbital after intravenous benzodiazepine achieved clinical seizure control except for three patients. Two of these were cases of epilepsy syndromes (tuberous sclerosis complex and Lennox-Gastaut syndrome) with intractable seizures already maintained on other ASMs. Oral phenobarbital was administered orally for acute repetitive seizures, but initially had poor seizure control; thus, the maintenance doses were increased. Seizure reduction to 50% from baseline was noted 4 days post loading, and their serum levels at 48 hours showed slightly higher serum levels (46 and 51 μg/mL). The third patient was a previous case of status epilepticus who already had seizure control with three other ASMs with maximal doses before having repetitive seizures. He was given a single dose of 15 mg/kg oral phenobarbital, and repetitive seizures persisted over the next hour before clinical control was achieved. Phenobarbital oral loading was given to 13 patients after intravenous levetiracetam (40%), phenytoin (20%), or valproic acid (4%) was unable to achieve adequate seizure control despite maximum doses. After administration of oral phenobarbital, clinical seizure control was achieved in 10 patients (70%). Of the patients with persistent clinical seizures, one needed a subsequent increase in the maintenance dose; one was given another ASM (oxcarbazepine), and the other had no subsequent episode after the initial seizure recurrence, showing adequate seizure control after oral phenobarbital.
The acute adverse effects of phenobarbital include rashes, hypersensitivity reactions, somnolence, drowsiness, and acute liver dysfunction [10,18]. Drowsiness was the most commonly reported adverse effect in 18 patients and was noted to be transient, with recovery within 24 hours. Four patients (16%) had an associated decreased sensorium post-loading. Two of them had concomitant pneumonia requiring intubation. One was a case of aqueductal stenosis that eventually needed a ventriculoperitoneal shunt, and the other was a case of a posterior fossa mass that loped decreased sensorium after the second dose of oral loading was given. Improvement was noted after transiently withholding subsequent maintenance doses of phenobarbital for 24 hours before resumption. Most of the adverse effects documented in these patients depended on their comorbidities at the time of seizure occurrence. These findings are similar to those of previous studies of oral phenobarbital loading in children and neonates [8,9]. Liver function measures were only available in three patients. The two patients with elevated liver enzymes had a concomitant liver injury; one had jaundice on admission, while the other had a vitamin K deficiency. Similarly, phenobarbital in this setting did not directly cause acute liver injury after the oral loading doses were given. A comparison between the two dosing regimens in terms of these adverse effects also showed no significant difference between the two groups.
This study was limited due to its retrospective nature and small sample size. Because of the short interval for study inclusion and the non-routine phenobarbital serum monitoring, the study participants were limited. A review of patients’ medical records would also be unable to fully document all the adverse events that may occur in these patients. A prospective and broader-spectrum study is thus recommended.
This study showed that oral loading of phenobarbital at a dose of 15 to 20 mg/kg/day given as a single dose or in two divided doses is an effective and safe method for achieving serum therapeutic effects within 24 hours after oral loading. This serum level was maintained and increased with maintenance doses. Children were able to tolerate this method with minimal and transient adverse reactions. This practice may thus be a helpful alternative at centers without access to intravenous phenobarbital or other newer ASMs.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JGDF, MMGV, and CMCU. Data curation: JGDF. Formal analysis: JGDF. Methodology: JGDF. Project administration: JGDF. Visualization: JGDF. Writing-original draft: JGDF. Writing-review & editing: JGDF, MMGV, and CMCU.