Frontal Lobe Epilepsy in a Pediatric Population: Characterization of Clinical Manifestations and Semiology
Article information
Abstract
Purpose
Frontal lobe epilepsy (FLE) has various clinical presentations depending on the anatomy involved. Seizures are brief and can mimic psychiatric conditions, and patients often cannot describe the aura. Therefore, it is difficult to characterize the semiology, especially in pediatric patients. This study investigated the characteristics of pediatric FLE.
Methods
We retrospectively reviewed the data of pediatric patients with FLE who underwent long-term video-electroencephalography (EEG) monitoring between January 2010 and June 2020. Patients’ demographic data, seizure-related clinical presentations, semiology, brain magnetic resonance imaging (MRI), and EEG data were analyzed.
Results
Fifty-six patients were included (31 males, 25 females). The age of seizure onset varied from 1 month to 14 years (mean±standard deviation, 6.1±4.4 years). Seizures were classified into nine categories, including focal tonic (30/56), aura (22/56), hypermotor (17/56), focal clonic (15/56), versive (13/56), and bilateral asymmetric tonic (4/56). Seventeen patients (30.4%) had abnormal MRI results, including focal cortical dysplasia, heterotopic gray matter, and neuroepithelial tumors. Ictal EEG changes were commonly observed in the dorsolateral premotor and central cortices. In focal tonic seizures, EEG changes often originated in the premotor cortex. The location of the lesions on MRI and EEG coincided in six cases.
Conclusion
In pediatric FLE, various seizure types occur depending on the ictal anatomic origin, and individual patients had multiple semiologies. Brain MRI was normal in two-thirds of patients, and interictal EEG did not reveal epileptiform discharges in approximately 25%. Semiology reported on the basis of home videos and interictal EEG will help localize the ictal onset zone.
Introduction
Frontal lobe epilepsy (FLE) has a difficult-to-understand and complex seizure semiology, which depends on the location of the epileptogenic area [1-6]. The frontal lobe is large and has several combined functions [1,3]. The epileptic propagation of FLE is complex and rapid; therefore, it is often difficult to predict the localization of ictal onset in clinical practice [4-8].
Several previous studies involving adult patients have described the semiological seizure characteristics of FLE as follows: very short duration, sudden onset and offset, stereotypic form, a tendency for clustering, nocturnal disposition, and rapid secondary generalization [2,9-15]. Because the frontal lobe is involved in not only motor function, but also in language and emotional control, the ictal appearance of FLE can present as psychiatric symptoms such as fearful feelings or paranoid delusions [1,7,16]. FLE is often misdiagnosed as pseudoseizures related to psychiatric conditions [7,9,16-20].
The few studies focusing on FLE in the pediatric population have revealed that frontal lobe seizures in children differ from those of adults [1,2,9,21-23]. Therefore, the semiological characteristics of pediatric FLE require definition using ictal data, which could provide much-needed information for clinicians in terms of diagnosis and treatment. The aim of this study was to identify the semiological characteristics of FLE in pediatric patients using long-term video electroencephalography (EEG) data.
Materials and Methods
1. Patients
We retrospectively reviewed the medical records of pediatric patients admitted to the Samsung Medical Center, Seoul between January 2010 and June 2020. During that period, 71 patients were diagnosed with FLE. Among them, patients with ictal events were included as subjects of this study. The inclusion criteria were as follows: (1) patients who underwent long-term video EEG monitoring; (2) patients under 18 years of age at the time of the long-term video EEG recordings; (3) patients who had clinical seizures during monitoring; and (4) patients with a final diagnosis of FLE. Autosomal dominant nocturnal FLE and genetic FLE were not clinically suspected in any patients. No patients underwent genetic studies, such as a next-generation sequencing-based gene panel study.
2. Data collection
Data on clinical variables, such as demographics, age of onset, seizure semiology, EEG, brain magnetic resonance imaging (MRI) findings, and anti-seizure medications used were collected. The seizure types were described using the semiological seizure classification proposed by Luders et al. [24] and Dugan et al. [25]. When describing patient seizure types, we included both the medical history and the seizure semiology confirmed during long-term video EEG monitoring at the time of admission.
In this study, we summarized the characteristics, including demographics, medical history, EEG, and neuroimaging findings, of pediatric FLE patients who underwent video EEG monitoring, with a focus on describing the seizure semiology. Furthermore, we analyzed the clinical manifestations of 10 patients with psychic auras to identify any common aspects.
3. Ethics
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB file no. 2022-04-183). The requirement for informed consent from patients was waived because of the retrospective nature of this study.
4. Localization of EEG
We classified the frontal lobes into the dorsolateral, mesial, and basal frontal lobes based on the anatomical locations, and the dorsolateral cortex was subdivided into the prefrontal, premotor, and central cortices. When electrodes were attached according to the international 10 to 20 system, the corresponding position of each electrode was checked. Based on this criterion, the Fp1 and Fp2 electrodes were classified as corresponding to the prefrontal cortex, the F3 and F4 electrodes as being placed on the premotor cortex, the C3 and C4 electrodes as corresponding to the central cortex, and the Fz and Cz electrodes as being placed on the mesial frontal cortex.
Results
1. Clinical manifestations
In total, 56 patients (25 females, 31 males) met the inclusion criteria. The age of onset ranged from 1 month to 14 years (mean, 6.1±4.4 years) (Table 1). Seventeen patients (30.4%) had a medical history of neurological conditions such as febrile seizures (5/56, 8.9%), developmental delay (4/56, 7.1%), intellectual disability (4/56, 7.1%), encephalitis (2/56, 3.6%), and cerebral hemorrhage (2/56, 3.6%). The number of patients who underwent monotherapy and polytherapy was 12 (21.4%) and 34 (60.7%), respectively.
2. Seizure semiology features
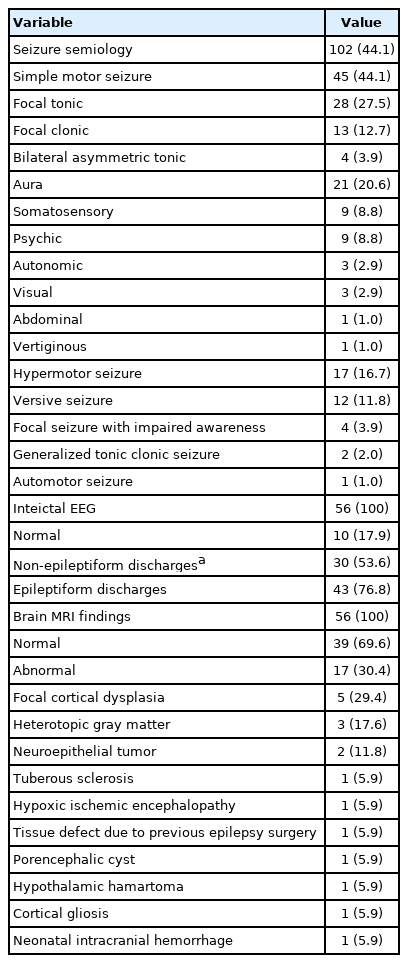
Based on patients’ medical records and ictal events during the long-term video EEG monitoring, we categorized seizures into nine categories according to the semiological seizure classification: focal clonic, focal tonic, asymmetric tonic, hypermotor, versive, dialeptic, automotor, generalized tonic clonic, and aura (Table 2). Auras were further classified as somatosensory, psychic, autonomic, visual, abdominal, or vertiginous. If there was more than one semiology in a single patient, each seizure type was counted. The 56 patients showed 102 seizure types in nine categories. Simple motor seizure (specifically, focal tonic seizure) was the most common type (45/56, 80.4%), followed by aura (21/56, 37.5%). Nine patients had somatosensory auras (9/56, 16.1%), and nine had psychic auras (9/56, 16.1%).
3. EEG
Interictal EEG was normal in 10 patients (17.9%) (Table 2). Twenty patients had interictal epileptiform discharges with normal background activity (35.7%); three patients had only non-epileptiform discharges, such as regional slow waves or generalized slow waves (5.4%) without any epileptiform discharges. Both epileptiform and non-epileptiform discharges were observed in 23 patients (41.0%). The ictal rhythm showed various forms such as repetitive spikes or sharp waves, fast activity, or rhythmic alpha or theta activity. The ictal onset zones were the left, right, both, or midline frontal or central areas.
4. Neuroimaging
Thirty-nine patients (69.6%) had normal structural brain MRI findings. Abnormal structural lesions were observed in 17 patients (30.4%) (Table 2). Focal cortical dysplasia was detected in five patients (5/17, 29.4%) (Fig. 1). Other abnormal brain MRI findings were heterotopic gray matter (n=3, 17.6%), neuroepithelial tumor (n=2, 11.8%), tuberous sclerosis complex (n=1), hypoxic ischemic encephalopathy (n=1), tissue defect due to previous epilepsy surgery (n=1), porencephalic cyst (n=1), hypothalamic hamartoma (n=1), cortical gliosis (n=1), and neonatal intracranial hemorrhage (n=1).
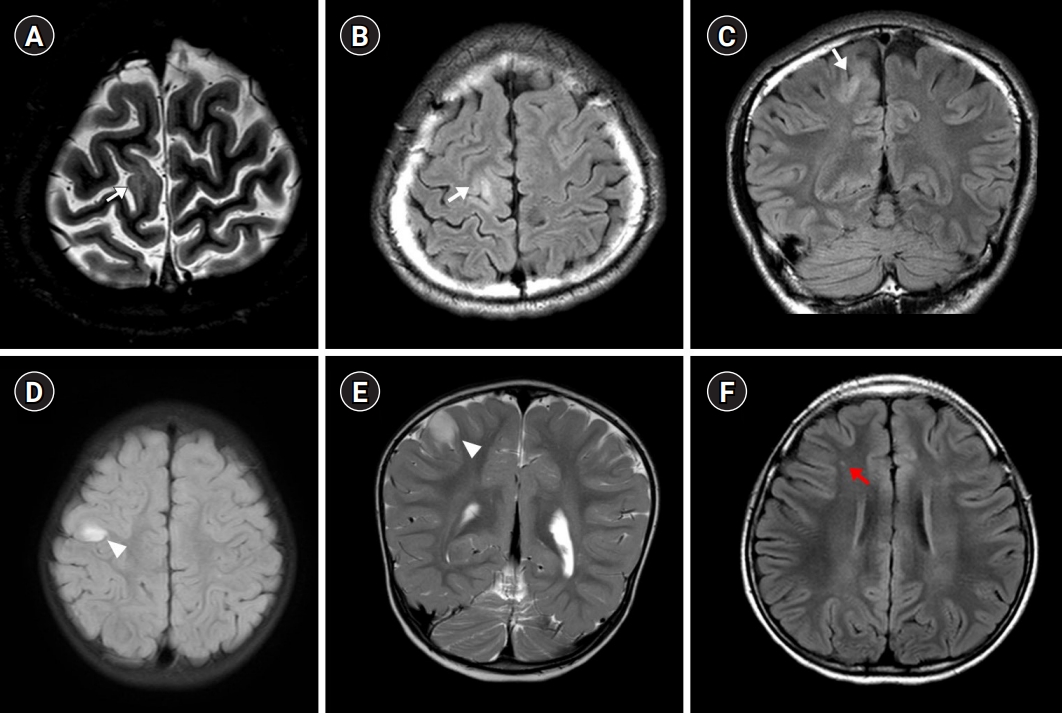
Axial T2-weighted (A) and fluid attenuated inversion recovery (FLAIR) (B) images and a coronal FLAIR (C) image demonstrate high signal intensity (white arrow) in the right frontal lobe, suggesting focal cortical dysplasia. Axial FLAIR (D) and coronal T2-weighted (E) images show high signal intensity (white arrowheads) in the right precentral gyrus, suggesting a neuroepithelial tumor. An axial FLAIR image (F) shows high signal intensity (red arrow) in the right frontal lobe, suggesting cortical gliosis.
5. Analysis of seizure semiology
1) Seizure frequency and types
Based on the medical records, 39 patients had daily seizures, nine had weekly seizures, and eighth had monthly seizures. The average period of hospitalization for the 56 patients was approximately 2.6 days. The patients experienced 102 types of seizures in nine categories during hospitalization, and a total of 641 seizures were observed. About 1.8 types of seizures were observed per patient, and an average of 4.3 seizures were observed per day. Ten patients had nocturnal seizures, and none had clustering seizures.
2) Ictal onset zone and seizure semiology
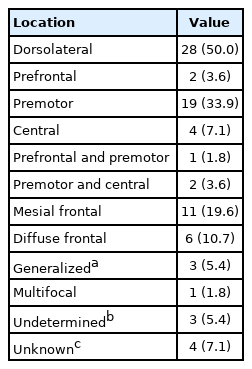
Considering the division of the frontal lobe into the dorsolateral, mesial, and basal frontal lobes and further the subdivision of the dorsolateral cortex into the prefrontal, premotor, and central cortices, we classified the ictal onset zone observed for each patient (Table 3). The dorsolateral cortex was the most common (28/56, 50.0%) onset zone (premotor cortex: 19/56, 33.9%; central cortex: 4/56, 7.1%; and prefrontal cortex: 2/56, 3.6%). Eleven patients (19.6%) had seizures originating in the mesial frontal lobe. Six patients (10.7%) had diffuse frontal lobe seizure origins (in the dorsolateral and mesial frontal lobes).
The ictal onset zones of the 102 types of seizures were also analyzed based on the four types of semiology commonly observed based on ictal EEG (Table 4). Focal tonic seizures commonly originated in the premotor cortex (6/28 patients). In addition, mesial frontal cortex (5/28) and diffuse/non-localized onset (4/28) were frequently observed. When focal clonic seizures occurred (n=13), the EEG changes were diffuse/non-localized in six patients and localized to the premotor area in three patients. Hypermotor seizures (n=17) were accompanied by EEG changes in the prefrontal cortex (4/17) and premotor cortex (3/17). Although auras revealed no EEG changes in most cases (8/21), psychic auras often showed EEG changes. The ictal onset zone for psychic auras was the prefrontal cortex in one case, the premotor cortex in two cases, the mesial frontal lobe in three cases, and an undetermined location in one case; there was no change in two cases.
3) Correlation between anatomic abnormalities and the ictal onset zone
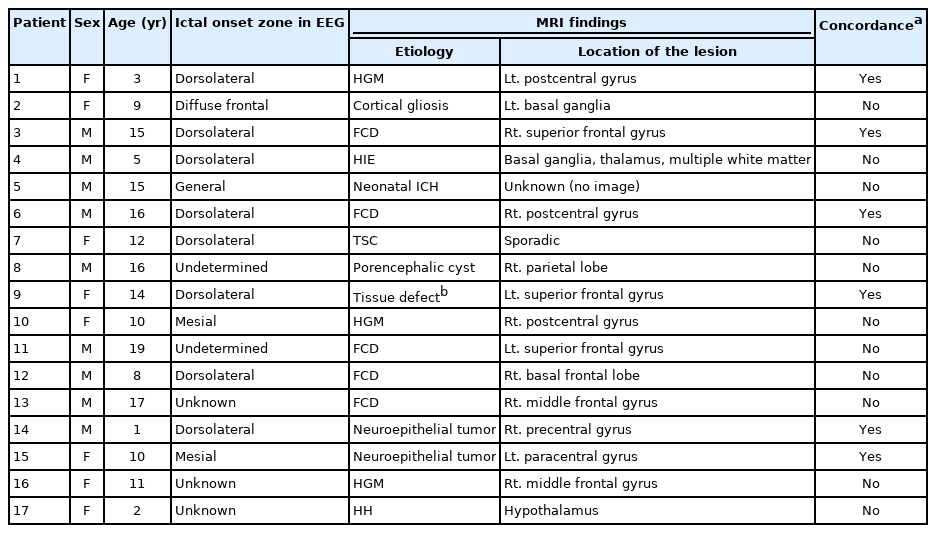
In the analysis of the ictal onset zones of the 17 patients with anatomical abnormalities on brain MRI, the most common localization of onset was observed to be in the dorsolateral frontal lobe (8/17) (Table 5). The ictal onset zone was in the mesial frontal lobe (n=2), diffuse in the frontal lobe (n=1), and diffuse/non-localized (n=1). Three cases showed no EEG changes, and in two cases, the ictal onset zone could not be determined (diffuse background attenuation). The location of the lesion on brain MRI coincided with the ictal onset zone on EEG in six cases. The lesions were focal cortical dysplasia in two cases, neuroepithelial tumor in two cases, heterotopic gray matter in one case, and tissue defect due to previous epilepsy surgery in one case. In the six patients with concordance between the location of the anatomic lesion on MRI and the EEG changes, the ictal onset zone was the dorsolateral frontal lobe in five cases and the mesial frontal lobe in one case.
4) Patients with psychic auras
There were 10 patients with psychic auras (Table 6). Nine of these patients showed psychic auras during long-term video EEG monitoring. One patient did not have this type of seizure during hospitalization, but had them habitually otherwise (listed as patient number 10 in Table 3). Patients complained of fears such as a feeling of being squeezed in the throat, being somewhere else, being sucked into something, or everyone disappearing. EEG changes were observed in eight of the nine patients, mainly in the frontal or central areas, when they had a psychic aura. Only one patient showed no EEG changes. Patients with psychic auras had several types of motor seizures and other types of auras as well, such as autonomic or visual auras. One patient (number 6) showed a structural abnormality on brain MRI (heterotopic gray matter in the right periventricular white matter) (Fig. 2). In this patient, ictal changes on EEG were observed in the midline frontocentral areas.
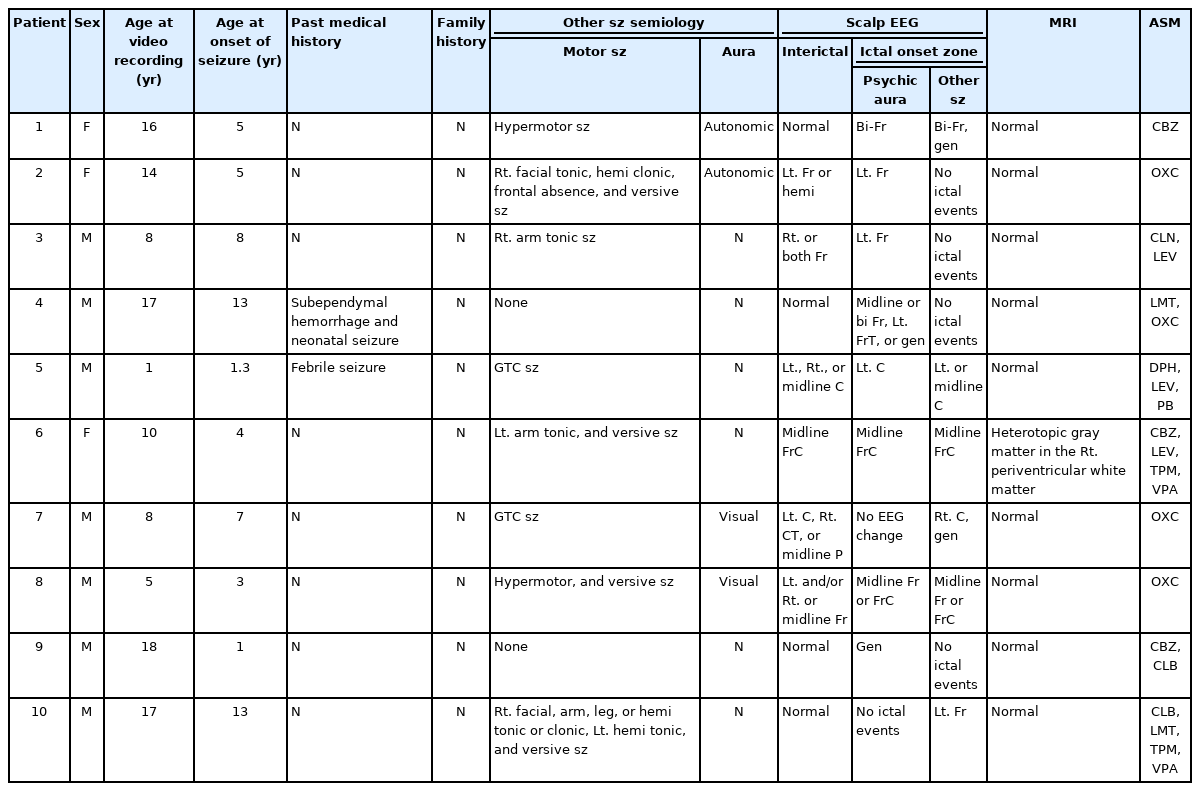
Clinical features, EEG findings, neuroimaging, and ASM history of 10 patients presenting with psychic auras (patient 10 did not show psychic auras during hospitalization)
Discussion
This retrospective study describes the characteristics of pediatric patients with FLE based on an analysis of their actual seizures using long-term video EEG monitoring. The seizure semiology observed was diverse, and included simple motor seizures, hypermotor seizures, and auras. Only 17.5% (10/57) of patients had nocturnal seizures and no patient had clustering seizures in long-term video EEG monitoring, although many of them had nocturnal and clustering seizures in their history. The dorsolateral cortex was the most common ictal onset zone (28/56, 50.0%; premotor cortex: 19/56, 33.9%; central cortex: 4/56, 7.1%; prefrontal cortex: 2/56, 3.6%). Focal tonic seizures often began with EEG changes in the premotor or mesial frontal cortices. The lesion location on brain MRI matched the ictal onset zone on EEG in six patients.
In this study, we classified semiology into nine categories. Focal tonic seizures (50.0%), psychic auras (16.1%), and hypermotor seizures (30.4%) were the most frequent categories. Interictal EEG was mostly normal, and was well-localized in 80.4% (45/56) and poorly localized in 19.6% (11/56) of patients (Table 3). In a previous study that included 22 pediatric patients with FLE, the semiology was as follows: focal tonic, focal clonic, hypermotor, versive, dialeptic, and psychic aura [1]. They reported that frontal lobe seizures were frequent and stereotypic and had rapid onset and offset. In their study, semiology was classified into six categories, among which hypermotor seizures (20/22) and psychic auras (15/22) were the most common. However, in our study, where semiology was classified into nine categories, simple motor seizures were the most common type (45/56, 80.4%), and hypermotor seizures were the third most common type (17/56, 30.4%). Auras were the second most common type (21/56, 37.5%), and psychic auras were found in only nine patients (16.1%). Compared with previous studies, this study was conducted with a larger number of patients. It is significant in that it analyzed all the ictal onset zones of 641 ictal events and correlated them with the semiology.
In our study, MRI revealed that 30.4% of patients (17/56) had various abnormalities, including focal cortical dysplasia (n=5), heterotopic gray matter (n=3), and neuroepithelial tumor (n=2). The abnormal findings of brain MRI in our study were different from those found in adult FLE patients. In a previous study including 36 adult patients, encephalomalacia (8/17, 47.1%) was the most common etiology, followed by traumatic brain injury, postoperative changes, malformation of cortical development (6/17, 35.3%), and vascular malformations (2/17, 11.8%) [9]. In a pediatric study, 14.3% to 100.0% of patients had focal cortical dysplasia [1,2], whereas in our study, only 8.9% (5/56) had focal cortical dysplasia. The abnormal brain MRI findings observed in the previous pediatric studies were only focal cortical dysplasia.
Long-term video EEG monitoring was mainly conducted to differentiate semiology in this study, but it has been used in previous studies for the purpose of presurgical evaluations of patients with anatomic abnormalities on MRI. Therefore, the proportion of focal cortical dysplasia among the patients was different.
Lesions located deep in the brain (9/17, 52.9%) had poor EEG correlations, whereas lesions near the cortex (8/17, 47.1%) tended to be well correlated (6/8). The reason for the poor correlation is thought to be that the epileptiform discharges become rapid and widely-propagating because of the rich connectivity between the frontal lobe and other lobes [9]. This proposal is supported by reports that the introduction of stereoelectroencephalography (sEEG) is helpful for confirming ictal spreading [4,8]. A study conducted by Bonini et al. [4] on 54 patients with adult FLE showed the process of clear localization using sEEG; seizures were categorized based on 31 ictal signs, and the electrode at which each seizure started was confirmed, leading to the finding of meaningful correlations between semiology and anatomy. Because our study was based on scalp EEG, there was a limitation in determining the precise ictal onset zone. In routine clinical practice, if the ictal onset zone cannot be precisely localized using scalp EEG, sEEG could be considered for surgical planning.
This study has several limitations. Because this study was focused on pediatric FLE patients who had ictal events during hospitalization, it did not represent all pediatric FLE patients. In addition, because the patients in this study showed various clinical characteristics, seizure semiology, EEG, and brain MRI findings, a limitation is that it was not possible to perform a statistical analysis because the number of patients in the groups to be compared was small.
In conclusion, this study summarizes the characteristics of pediatric patients admitted to our epilepsy monitoring unit and diagnosed with FLE. Brain MRI findings were normal in two-thirds of patients, and interictal EEG did not reveal epileptiform discharges in 23.2% of the patients. Because interictal EEG and brain MRI are often normal in patients with FLE, it is important to understand the characteristics of frontal lobe seizures in order to diagnose FLE and differentiate these seizures clinically. To determine the ictal onset zone in the patients already diagnosed with FLE, it is necessary to understand the functional anatomy of the frontal lobe and seizure semiology and to confirm the ictal EEG through long-term video EEG. Reports of semiology on the basis of home videos obtained by parents and interictal EEG will be the first steps in localizing the ictal onset zone. Thereafter, long-term video EEG may be helpful for further localization. For FLE with a nonlocalized structural etiology, sEEG will be helpful for defining the ictal onset zone.
Notes
Jeehun Lee is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: DL, JL, and JL. Data curation: DL. Formal analysis: DL and JL. Project administration: DL. Visualization: DL, JL, and JL. Writing-original draft: DL. Writing-review & editing: JL and JL.