Effectiveness of Intravenous Immunoglobulin Therapy for Pediatric Viral Encephalitis
Article information
Abstract
Purpose
Intravenous immunoglobulin (IVIg) is considered as a first-line therapy for autoimmune encephalitis. However, its effectiveness in viral encephalitis has yet to be evaluated. Therefore, we investigated the effectiveness of IVIg therapy for pediatric viral encephalitis.
Methods
We retrospectively reviewed the records of 35 pediatric patients who were hospitalized with confirmed or suspected viral encephalitis. Twenty patients (57.1%) were treated with IVIg in conjunction with conventional therapy (IVIg-treated group), and 15 patients (42.9%) were treated with conventional therapy (non-IVIg-treated group). We compared the clinical characteristics of the groups at admission and their clinical outcomes.
Results
Compared to the non-IVIg-treated group, the IVIg-treated group had more critical clinical features at admission, with a lower score on the pediatric Glasgow Coma Scale (mean±standard deviation, 9.1±2.3 vs. 10.8±2.7, P=0.025), longer fever duration (3.5±2.2 days vs. 1.8±1.1 days, P=0.022), and higher incidence of magnetic resonance imaging abnormalities (14/19 [73.7%] vs. 3/15 [20.0%], P=0.002). Nevertheless, the clinical outcomes of the IVIg-treated group were comparable to those of the non-IVIg-treated group in terms of mortality rate (1/20 [5.0%] vs. 0/15 [0%]), neurological deficits at discharge (2/20 [10.0%] vs. 1/15 [6.7%]), and occurrence of epilepsy (5/20 [25.0%] vs. 2/15 [13.3%]). Fourteen (70.0%) and 13 (85.7%) patients in the IVIg-treated and the non-IVIg-treated groups, respectively, achieved complete recovery without any neurological complications.
Conclusion
IVIg may be considered as a potential immunomodulating agent when treating critical pediatric viral encephalitis to improve neuropsychological outcomes.
Introduction
Encephalitis is a clinical syndrome resulting from the inflammation of the brain parenchyma [1,2]. It presents with neurological dysfunctions, such as altered consciousness. The global incidence of encephalitis was reported to be 4.3 million cases in 2015, of which 150,000 patients had died [3,4]. Encephalitis is caused by direct invasion of microorganisms, such as viruses, bacteria, or fungi into the brain parenchyma. Alternatively, it can be caused by overactivation of the host immune system in the brain due to post-infection processes, such as acute disseminated encephalomyelitis, or noninfectious conditions such as anti-N-methyl-D-aspartate receptor encephalitis [2]. In fact, the cause of encephalitis is unknown in approximately half of the cases [5]. A recent report revealed that the prevalence of autoimmune encephalitis is similar to that of infectious encephalitis in adults [6]. In children, however, the most common cause of encephalitis is an infection by microorganisms [7]. Viral encephalitis accounts for 20% to 50% of the cases with a known cause [5]. Herpes simplex virus infection is the most common sporadic cause of viral encephalitis, accounting for 50% to 75% of identified viral cases. Varicella-zoster virus (VZV), enterovirus, Epstein-Barr virus, and cytomegalovirus (CMV) are also common causes [5]. The prognosis of viral encephalitis, including mortality and neurological complications varies according to the causative pathogens [1,5].
The basis of therapy for viral encephalitis is the use of antiviral agents, such as acyclovir. The mortality rate of patients with herpes simplex virus encephalitis has sharply decreased from 70% to 10%–20% since the introduction of acyclovir in the 1970s [8]. However, despite acyclovir therapy, 69% to 89% of survivors still suffer from a wide range of neuropsychological sequelae [9,10]. Moreover, there is a lack of evidence on the effectiveness of specific antiviral agents for other viral causes [1]. Hence, the application of additional treatment strategies other than conventional antiviral agents is highly desirable for the treatment of viral encephalitis.
During viral infection, the host’s immune response is crucial to eliminate invading viruses. However, since excessive host immune response can be harmful to the host cells, immunomodulation is also important when treating viral infection. Intravenous immunoglobulin (IVIg) is a well-known example of an immunomodulating agent [11]. It has been used in a variety of systemic diseases, such as immunothrombocytopenia and Kawasaki disease [12]. It has also been used when treating inflammatory neurological diseases, such as Guillain-Barré syndrome [13]. Although IVIg is considered as a first-tier therapy for autoimmune encephalitis, there is thus far a lack of studies on its effectiveness in viral encephalitis. This study aimed to evaluate the potential efficacy of IVIg therapy in the treatment of viral encephalitis.
Materials and Methods
1. Participants
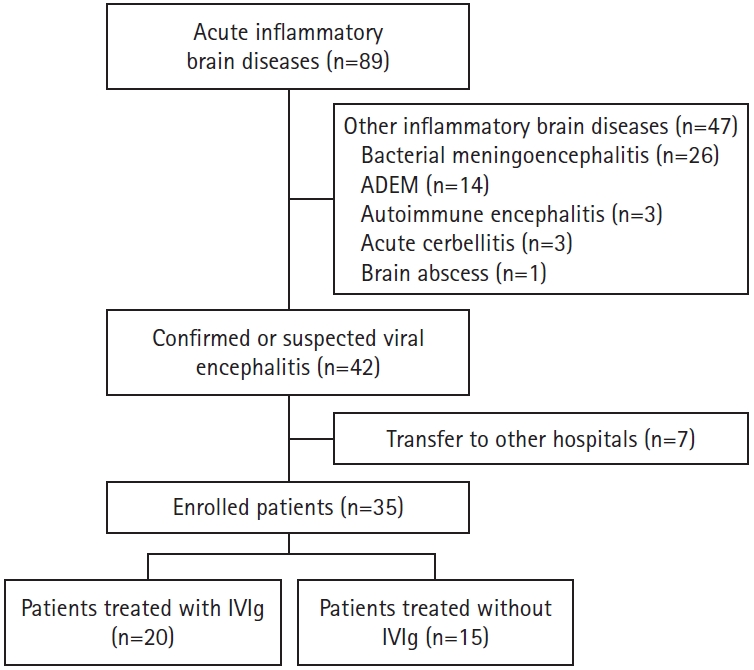
We retrospectively reviewed the records of 89 pediatric patients with acute inflammatory brain diseases who were admitted to the Ulsan University Hospital between January 2012 and December 2019. Among the 89 patients, 26 had bacterial meningoencephalitis, 14 had acute disseminated encephalomyelitis, three had autoimmune encephalitis, three had acute cerebellitis, and one had a brain abscess. These 47 patients were excluded from the study (Fig. 1). The remaining 42 patients had confirmed or suspected viral encephalitis. Seven of these were transferred to other hospitals and were thus excluded from the study. Finally, 35 patients with confirmed or suspected viral encephalitis were included in the analysis. All patients included in this study were otherwise healthy before the appearance of the cause of admission and had no history of immunoglobulin therapy.
2. Methods
In this study, we applied modified diagnostic criteria for encephalitis, adapted from the International Encephalitis Consortium [2]. When the following major and minor criteria were fulfilled, patients were diagnosed with encephalitis. Major criteria included altered mental status for at least 12 hours (defined as decreased or altered level of consciousness, lethargy, or altered personality) with no alternative cause identified. Minor criteria were considered to be fulfilled when two or more of the following six criteria were met: (1) fever ≥38°C within the 72 hours before presenting with altered consciousness; (2) generalized or partial seizures not fully attributable to a preexisting seizure disorder; (3) new onset of focal neurological deficits; (4) cerebrospinal fluid (CSF) pleocytosis; (5) abnormality of brain parenchyma in magnetic resonance imaging (MRI) suggestive of encephalitis; and (6) abnormality on electroencephalography (EEG) indicating encephalitis and not attributable to another cause. In this study, a diagnosis of viral encephalitis was made based on clinical features and various test results, including MRI, EEG, and routine CSF analysis; and reasonable exclusion of other causes of encephalitis, regardless of confirmatory diagnostic test results.
To determine the etiology of encephalitis, specific tests were performed based on the clinical manifestations and initial test results, such as the previously mentioned MRI, EEG, and routine CSF analysis. All patients underwent routine CSF analysis that included white blood cell count, total protein level, and bacterial culture. CSF pleocytosis was defined as the presence of ≥5 white blood cells/mm3 in CSF. To identify the causative viruses for encephalitis, the following analyses were performed: (1) CSF polymerase chain reaction (PCR) for enterovirus (n=32), herpes simplex virus (n=23), VZV (n=8), and CMV (n=1); (2) CSF mycoplasma-specific immunoglobulin M (IgM) tests (n=6); (3) serological tests for measles (n=4), Chlamydia pneumoniae (n=3), mumps virus (n=3), rubella virus (n=2), parvovirus B19 (n=2), and toxoplasma (n=1); (4) PCR for enterovirus in stool samples (n=12); (5) rotavirus antigen test in stool samples (n=3); and (6) PCR for CMV in urine samples (n=2). For genome detection and genotyping of enterovirus, real-time quantitative PCR using the Taq-Man probe technology was attempted on stool and CSF samples obtained from 12 patients. Genotyping of the enterovirus was conducted at the Division of Enteric and Hepatitis Viruses, Korea Centers for Disease Control and Prevention (Osong-eup, Cheongwon, Korea).
MRI was performed on either a 3.0 Tesla system (Intera Achieva, Philips, Best, The Netherlands) or a 1.5 Tesla system (Intera Achieva, Philips). The protocol included T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and contrast-enhanced T1-weighted and FLAIR imaging. MRI was performed in all patients except one (97.1%). This patient could not undergo MRI because of her critical condition due to enterovirus 71 brainstem encephalitis. In this study, meningeal enhancement without brain parenchymal lesion was not considered as an abnormal MRI finding. EEG was performed in 27 patients (77.1%). Eight patients (22.9%) who had suspected or confirmed enterovirus 71 brain stem encephalitis did not undergo EEG.
The participating patients were divided into two groups on the basis of the inclusion of IVIg in their therapy regime. IVIg treatment was selectively used in patients with more severe neurological manifestations, according to the pediatric neurologist’s judgment instead of specifically defined criteria. Corticosteroids were not used in any patient as an acute treatment for encephalitis. Twenty patients (57.1%) were treated with IVIg (2 g/kg) in conjunction with conventional therapy (IVIg-treated group), whereas 15 patients (42.9%) were treated with conventional therapy only (non-IVIg-treated group). Conventional therapy included intravenous acyclovir and/or antibiotic therapy and conservative management. We compared the groups for manifestations at admission, including clinical features (altered consciousness, fever, seizure, and focal neurologic signs) and severity (Pediatric Glasgow Coma Scale score, duration of altered consciousness, duration of fever, presence of status epilepticus, and the need for treatment at the intensive care unit [ICU]), MRI and EEG findings, and CSF profiles. In addition, we compared the groups for the clinical outcomes, including interval from admission to recovery of consciousness, interval from admission to disappearance of fever, neurological deficits at discharge, occurrence of epilepsy after discharge, and mortality. Lastly, we evaluated in detail the characteristics of patients included in the IVIg-treated group.
This study was approved by the Institutional Review Board of Ulsan University Hospital (IRB No. 2020-04-043). Informed consent was waived by the board due to the study’s retrospective nature.
3. Statistical analysis
Data were analyzed using IBM SPSS version 21.0 (IBM Co., Armonk, NY, USA). The Pearson’s chi-square test was used to compare the groups for patients’ age, clinical symptoms, EEG abnormalities, the number of patients admitted to the ICU. The Fisher’s exact test was used to compare the presence of seizures, CSF pleocytosis, MRI abnormality, mortality, neurological deficits at discharge, and occurrence of epilepsy. The independent t-test was used to compare the duration of fever, duration of altered consciousness, and the number of seizures at admission. The Mann-Whitney U test was used to compare the sex of the patients, Pediatric Glasgow Coma Scale score, the number of patients who had status epilepticus at admission, and the interval from admission to disappearance of fever and recovery of consciousness. Continuous variables are reported as the mean±standard deviation. Differences with P<0.05 were considered statistically significant.
RESULTS
1. Patient demographics
The mean ages were 6.7±4.8 and 7.7±4.9 years in the IVIg-treated and the non-IVIg-treated groups, respectively. There were 13 (65.0%) males in the IVIg-treated group and 10 (66.7%) males in the non-IVIg-treated group (Table 1).
2. Comparison of clinical features and MRI, EEG, and laboratory findings between the groups at admission
A comparison between the groups for clinical manifestations and MRI, EEG, and laboratory findings at admission is summarized in Table 1. At admission, the IVIg-treated group had more critical clinical features than the non-IVIg treated group. The IVIg group had a lower Pediatric Glasgow Coma Scale score (9.1±2.3 vs. 10.8±2.7, P=0.025) and longer duration of fever (3.5±2.2 days vs. 1.7±1.1 days, P=0.022). Moreover, the IVIg-treated group had higher incidence of MRI abnormalities (14/19 [73.7%] vs. 3/15 [20.0%], P=0.002). The IVIg-treated group tended to have a higher rate of ICU admission compared to the non-IVIg-treated group (13/20 [65.0%] vs. 5/15 [33.3%]), but this trend was not statistically significant (P=0.064). Nine patients (45.0%) had seizures in the IVIg-treated group at admission, whereas 12 patients (80.0%) had seizures in the non-IVIg-treated group (P=0.036). White blood cell counts in blood and CSF were higher in the IVIg-treated group than those in the non-IVIg-treated group, whereas C-reactive protein levels did not differ between the groups. No difference was observed in the incidence of fever, status epilepticus, focal neurological signs, CSF pleocytosis, and EEG abnormalities between the groups.
3. Comparison of clinical outcomes between the groups
A comparison between the groups for clinical outcomes is summarized in Table 2. Of the total 35 patients, only one patient (2.9%) included in the IVIg-treated group died. No difference was observed in the mortality (1/20 [2.9%] vs. 0/15 [0%], P=1.000), neurological deficits at discharge (2/20 [10.0%] vs. 1/15 [6.7%], P=1.000), and occurrence of epilepsy (5/20 [25.0%] vs. 2/15 [13.3%], P=0.672) between the groups. Also, there was no significant difference in the time from admission to recovery of consciousness (2.8±3.2 days vs. 3.0±4.9 days) and disappearance of fever (1.6±2.0 days vs. 1.5±3.1 days) between the groups. Fourteen (70.0%) and 13 (86.7%) patients achieved complete recovery without any neurological complications in the IVIg-treated and non-IVIg-treated groups, respectively.
4. Characteristics of the IVIg-treated group
1) Clinical features and laboratory, MRI, and EEG findings at admission
Clinical features and laboratory, MRI, and EEG findings of the 20 patients treated with IVIg at admission are described in detail in Table 3. The patients’ age ranged between 3 months and 15 years. The Pediatric Glasgow Coma Scale scores ranged between 4 and 13. Nine patients (45.0%) had seizures, which evolved into status epilepticus in two of the patients (22.2%). Four patients (20.0%) had focal neurological signs. One patient had ataxia, two had weakness of the right arm, and the other patient showed tremor of the tongue and the right leg after his consciousness had altered.

Clinical features and laboratory, magnetic resonance imaging, and electroencephalographic findings in 20 patients treated with intravenous immunoglobulin
Enterovirus 71 was detected in stool samples of six patients (30.0%). Herpes simplex virus was identified by a serological test (23- and 48-fold increase of herpes simplex virus-specific IgG titer along with positive result in IgM) in two patients (10.0%). The mean CSF white blood cell count was 87.4 cells/mm3 (range, 0 to 430). Sixteen patients (80.0%) had CSF pleocytosis. The mean protein level was 63.2 mg/dL (range, 16.3 to 149.0). Nine patients (45.0%) showed increased protein levels (>50 mg/dL).
Seven of the 19 patients (36.8%) who underwent MRI showed increased signal intensity in the posterior region of the brain stem and the bilateral cerebellar dentate nuclei on T2-weighted and FLAIR imaging. Six patients (31.6%) showed increased signal in the cerebral cortex. Four patients (21.1%) showed only leptomeningeal enhancement without parenchymal lesions. One patient (5.3%) had lesions in both basal ganglia, and one patient (5.3%) displayed normal images.
Nine of the 13 patients (69.2%) who were examined by EEG showed an abnormality in the test. All nine patients had background slowing on the EEG. One patient (7.7%) had focal spikes. Four patients (30.8%) had normal EEG findings.
2) Clinical outcomes and complications
Clinical outcomes of the IVIg-treated group are described in detail in Table 4. A dose of 2 g/kg of IVIg was administered for 2 to 5 days to 18 patients (90.0%). One patient received 2.4 g/kg of IVIg for 6 days, and one patient, who expired a day after admission, received 1 g/kg of IVIg for one day. The mean interval between admission and commencement of IVIg treatment was 24.6 hours (range, 4 to 100). The mean time from IVIg administration to recovery of consciousness was 3.0 days (range, 1 to 13). In four patients (20.0%), IVIg treatment was commenced after regaining consciousness. The mean interval between IVIg administration and disappearance of fever was 2.4 days (range, 1 to 6). In 10 patients (50.0%), IVIg treatment was commenced after the fever had already subsided. Thirteen patients (65.0%) were admitted to the ICU. The mean length of stay at the ICU was 4.2 days (range, 1 to 16). Two patients (10.0%) required ventilator management during the time at the ICU. The mean length of hospital stay was 15.5 days (range, 1 to 57). None of the patients had adverse effects from the IVIg therapy during the treatment.
One patient (5.0%) with enterovirus 71 brainstem encephalitis died due to pulmonary hemorrhage, acute heart failure, and shock 17 hours after admission. One patient (5.0%) experienced pneumonia and sepsis during hospitalization. These were completely resolved with antibiotic therapy. Two patients (11.1%) showed neurological deficits at discharge. One patient had weakness in his right arm. The other one had a tremor in his right leg, but this tremor had completely subsided by the time of a follow-up visit to the outpatient clinic two months later. Fourteen patients (70.0%) achieved complete recovery without any neurological complications. Anti-epileptic medication was prescribed to six patients (30.0%) at the time of discharge. Five patients (25.0%) were newly diagnosed with epilepsy after discharge.
Discussion
In the present study, at admission, although the IVIg-treated group had a more critical clinical condition than the non-IVIg-treated group, the clinical outcomes of both groups were comparable. Only one (5.0%) of the 20 patients treated with IVIg in conjunction with acyclovir had died, one (5.0%) had residual neurological deficits, and five patients (25.0%) were newly diagnosed with epilepsy. Otherwise, 14 patients (70.0%) achieved complete recovery without any neurological complications. Considering that the mean Pediatric Glasgow Coma Scale score in the patients treated with IVIg was approximately 9 at admission, 14/19 patients (73.7%) had brain parenchymal lesions on MRI, and 13 patients (65.0%) were treated in the ICU, the mortality and morbidity results look promising. It is, however, hard to conclude whether IVIg treatment was effective for the treatment of pediatric viral encephalitis because this study was a retrospective observational study at a single center. In addition, the study included only a small number of patients with encephalitis caused by a diversity of viruses, including herpes simplex virus, enterovirus 71, and, most commonly, unidentified viruses. Nevertheless, this study implies that IVIg could at least be considered as an additional medication in support of the conventional antiviral agents to improve the clinical outcome of patients with critical viral encephalitis.
During acute viral infection, immune system is activated through pathogen-sensing by various pattern-recognition receptors, such as Toll-like receptors. Innate immune cells, such as macrophages and dendritic cells, produce interferon-α and -β via the nuclear factor-κB and the interferon regulating factor signaling pathway [14]. The release of proinflammatory cytokines and chemokines then activates the innate immune and adaptive immune responses. Although cytokines and chemokines are produced mainly by immune cells, during acute viral infection, they are also produced in the central nervous system by the microglia and astrocytes [15-17]. The chemokines produced during viral infection induce the migration of leukocytes from the vascular lumen to the infected brain parenchyma, across the blood-brain barrier. This migration is achieved through the adhesive molecular interactions between the leukocytes and the endothelial cells [18-20]. While the recruited immune cells play a key role in eliminating infected brain cells, they might also damage uninfected surrounding cells in the process. Hence, the immunomodulation is critical during the management of viral encephalitis. Indeed, in a study on herpes simplex virus encephalitis in mice, mortality was associated with the severity of inflammatory responses, but not the virus titer [21]. In Japanese encephalitis, inflammation contributed to disease severity by disrupting the integrity of the blood-brain barrier, inducing neuronal cell death, and inhibiting the proliferation and differentiation of neural progenitors [22].
IVIg has been used for the treatment of a broad range of autoimmune and systemic inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, immunothrombocytopenia, autoimmune hemolytic anemia, and Kawasaki disease [23]. In addition, it has been applied to the therapy of autoimmune and inflammatory neurological disorders, such as Guillain-Barré syndrome, myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, and autoimmune encephalitis [24]. Although IVIg has been used off-label for the treatment of encephalitis caused by enterovirus 71, Japanese encephalitis virus, and West Nile virus, there is a lack of clinical studies on the efficacy and safety of IVIg for viral encephalitis [25-27]. Since 2015, the first randomized controlled trial, has been conducted to evaluate the effect of IVIg treatment in pediatric encephalitis of any cause [28]. This clinical trial included 308 children (6 months to 16 years) across 30 hospitals in the United Kingdom. It planned to recruit patients over 42 months and conduct follow-up of each participant for 12 months post-randomization.
IVIg is prepared from pools of serum IgG, retrieved from 3,000 to 60,000 healthy blood donors. A cold ethanol precipitation step is used to enrich for the serum IgG fraction [29,30]. This step is followed by several viral inactivation, depletion of blood coagulation factors, and removal of IgG aggregates. Besides IgG, various amounts of other immunoglobulin isotypes, most notably IgA, can be found in the IVIg preparation [31].
Despite the widespread use and therapeutic success of IVIg, the mechanism of IVIg action is not well understood. IgG molecules have two functional domains, The F(ab′)2 fragment (antigen-binding fragment), which is responsible for antigen recognition, and the Fc fragment, which is crucial for activating the innate immune system [31]. In IVIg preparation, the F(ab′)2 fragments of the IgG molecules recognize and bind not only to foreign antigens but also to a wide range of self-antigens. F(ab')2 fragment-dependent mechanisms of IVIg activity include the killing of target cells by antibody-dependent cytotoxicity, blocking cell interactions by inhibiting cell-surface receptors, such as CD95 and CD95 ligands, eliminating anaphylatoxins, such as C3 and C5, and neutralizing cytokines and autoantibodies [31,32]. The Fc fragment of the IgG binds the family of Fcγ receptors (FcγR), which are broadly expressed by most cells of the innate immune system, including eosinophils, neutrophils, monocytes, and macrophages. In humans, the FcγR family consists of several activating receptors and one inhibitory receptor, FcγRIIB [33,34]. Fc‑dependent mechanisms of action include expanding the regulatory T cell population, blocking the binding of immune complexes to FcγR, modulating dendritic cell activation via activation of FcγRIII, and modulating the activating and inhibitory FcγR expression on innate immune cells and B cells [31].
In general, IVIg infusion is well tolerated. The common side effects of IVIg are mostly transient and mild. It includes flushing, headache, malaise, fever, chills, fatigue, and lethargy [35]. However, in some rare cases, serious side effects, such as renal failure, transfusion-related acute lung injury, thrombosis, and arrhythmia have been reported [35]. Early assessment of risk factors, slow infusion, and premedication may lessen side effects. Fortunately, in this study, no side effects were observed in the patients treated with IVIg.
As briefly mentioned above, this study has considerable limitations. First, in this study, to identify the etiology of encephalitis, different tests were selectively conducted among patients with encephalitis based on their clinical manifestations and initial test results instead of following an established diagnostic protocol to screen for all known etiologies of encephalitis, including autoimmune encephalitis. In addition, this study included patients with suspected viral encephalitis, a diagnosis made based on clinical, laboratory, and MRI features along with reasonable exclusion of other causes without confirmatory test results. This might lead to inclusion of misdiagnosed viral encephalitis. Second, IVIg treatment was selectively used in patients who showed more severe neurological conditions according to the treating physician’s judgment. No specific criteria were used for the initiation of this treatment. We, therefore, could not include suitable controls for appropriate outcome-comparison with the patients treated with IVIg. Third, as this study included only a small number of patients from a single institute, selection bias may have occurred. Fourth, in this study, the identified causative virus was mostly enterovirus 71. However, in 55% cases, no specific virus could be identified. This precludes the possibility of evaluating what kind of viral encephalitis responds effectively to IVIg therapy. Fifth, autoantibody tests for autoimmune encephalitis were performed only in a few patients who had clinical manifestations suggesting such an etiology, such as abnormal psychiatric behavior, cognitive dysfunction, and movement disorder. Lastly, in patients whose parents did not complain of their having any neuropsychological symptoms, testing for those diseases were not performed. Therefore, neuropsychological complications, such as attention deficit hyperactivity disorder and learning disability could be underestimated in this study.
In conclusion, this study demonstrated the relatively low mortality (5.0%) and morbidity (30.0%) rates in children with critical viral encephalitis who were treated with IVIg in conjunction with conventional treatment. None of the patients had adverse effects to the IVIg therapy. This suggests the possibility of using IVIg as a potential immunomodulating agent for the treatment of pediatric viral encephalitis, aiming to improve clinical outcomes. Although IVIg therapy was described in several studies on viral encephalitis [36,37], including enterovirus 71 and Japanese B encephalitis, to the best of our knowledge, this is the first Korean study to focus on the efficacy of IVIg therapy in patients with viral encephalitis. Well-designed randomized control trials with a larger number of participants and a longer duration of follow-up are needed to determine whether IVIg therapy can improve the neuropsychiatric outcome of patients with viral encephalitis. In addition, further investigations are required to identify the mechanisms of action of IVIg in viral encephalitis treatment.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: KYL. Data curation: JEB and KYL. Formal analysis: JEB and KYL. Funding acquisition: KYL. Methodology: KYL. Visualization: JEB. Writing-original draft: JEB and KYL. Writing-review & editing: KYL.
Acknowledgements
This work was supported by the 2019 Ulsan University Hospital Research Grant (UUH-2019-08). We thank the patients and their families for placing their trust in us.