Mirror Movements in a Pediatric Patient with a Basal Ganglia Germinoma
Article information
Mirror movements (MMs) refer to involuntary movements in one limb that are performed simultaneously with voluntary movements in the other limb [1-4]. Although MMs are observed in normal children aged <10 years when the corpus callosum is maturing, pathological MMs may be noted in patients who have experienced cerebral infarction, Parkinson’s disease, tumors, or vertebral trauma [1-4]. Here, we report a 9-year-old boy with a germinoma in the right basal ganglia presenting with only MMs in the ipsilateral hand without other neurological problems.
A 9-year-old boy experienced difficulties in performing bimanual tasks, such as playing the piano and computer games, owing to involuntary movements of his right hand that had been first observed 3 months earlier. Prior to the onset of such movements, he had been healthy; his birth had been uneventful. He was born to non-consanguineous parents at a gestational age of 40 weeks with a birth weight of 3.92 kg. None of the family members presented with similar MMs or other neurological disorders. A neurological examination revealed no paresis or paresthesia. However, recurrent fisting of his left hand caused involuntary and clumsy fisting of his right hand.
The findings of other physical examinations, basic laboratory tests, chest roentgenography, and electrocardiography were all normal. Brain magnetic resonance imaging (MRI) performed on admission revealed a mass measuring 4.8×3.2 cm, with mixed cystic and solid components, in the right basal ganglia (Fig. 1A-D). Brain tractography image revealed disruption and posterior displacement of the fibers of the right corticospinal tract in the right frontal lobe; however, aberrant projections were not observed (Fig. 1E and F). Biopsy and pathological evaluation of the tissue from the brain lesion revealed that the lesion was populated by large tumor cells with clear cytoplasm and occasional distinct nucleoli (Fig. 2A). The tumor cells were interspersed with mature lymphocytes. The tumor cells were strongly positive for placental alkaline phosphatase and CD117 (c-Kit) in the immunohistochemical analysis (Fig. 2B). The lesion was diagnosed as a germinoma. The level of serum beta-human chorionic gonadotropin (β-hCG) was 61 mIU/mL (normal range, 0 to 5). No metastatic lesion was found on whole-spine MRI.
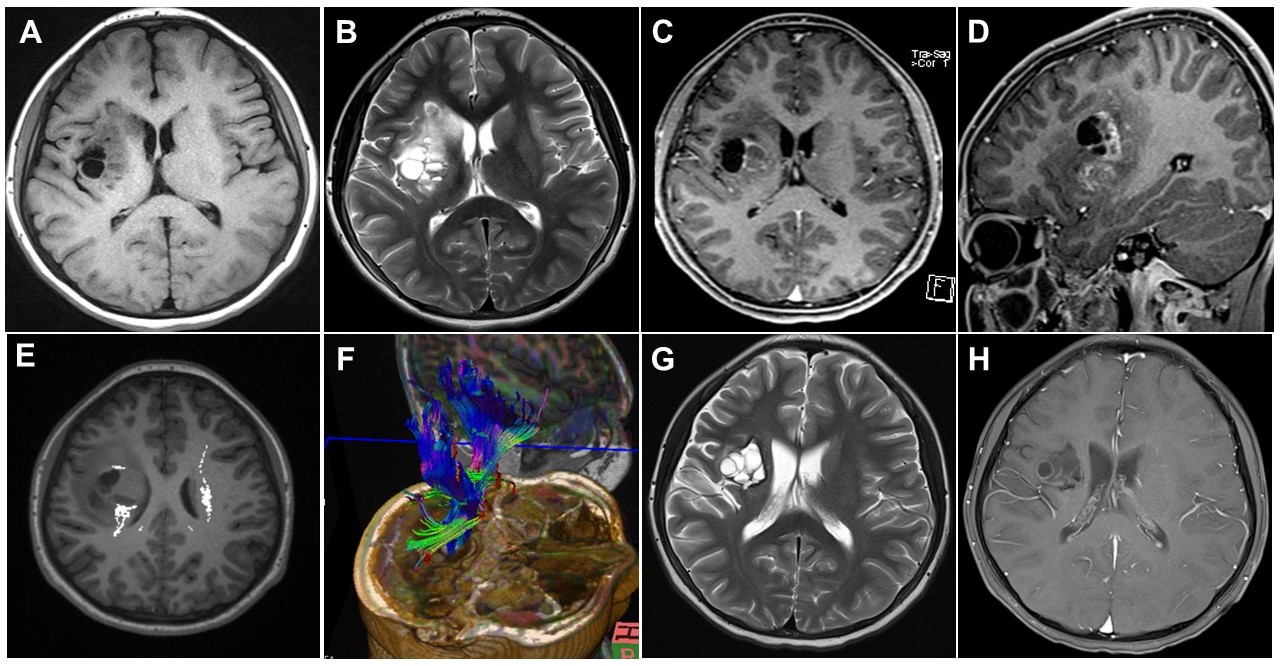
Brain magnetic resonance imaging (MRI) was initially performed for diagnosis (A–F) and 3 months after the first visit (G, H). Axial T1-weighted (A) and axial T2-weighted (B) images showing multiple cystic and solid masses with perilesional edema in the right basal ganglia. Axial (C) and sagittal (D) contrast-enhanced T1-weighted images showing enhancement in the cystic walls and solid portions. Diffusion-tensor images (E, F) showing disruption and posterior displacement of fibers of the right corticospinal tract in the right frontal lobe. The axial T2-weighted (G) and T1-weighted (H) contrast-enhanced images showing decreased tumor spread and decreased enhancement and cystic components.
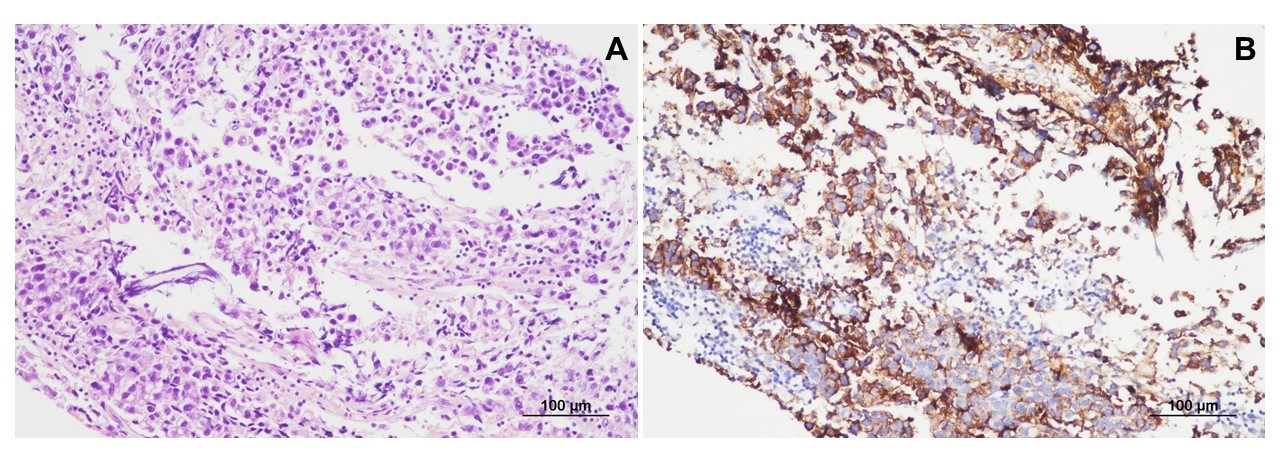
Histopathological findings for the germinoma. (A) The lesion comprises large tumor cells with clear cytoplasm and mature lymphocytes (hematoxylin and eosin staining, ×200). (B) The tumor cells are immunoreactive for placental alkaline phosphatase (immunohistochemical staining, ×200).
The patient received Korean Society for Pediatric Neuro-Oncology 081 (KSPNO 081) chemotherapy. During chemotherapy, the patient could perform bimanual tasks more comfortably with the gradual alleviation of the MMs in his right hand. At the end of chemotherapy, the serum β-hCG levels had decreased to 2 mIU/mL. The second brain MRI performed 3 months after the first visit showed a decrease in the spread of the tumor in the right basal ganglia (3.4×3.2 cm) and decreased enhancement and cystic components (Fig. 1G and H). The patient received radiotherapy at 1 month after the termination of chemotherapy. The MMs were still observed intermittently in his right hand, although they were mild.
A germinoma can develop in the pineal recess, suprasellar region, basal ganglia, and thalamus, and it can be associated with increased levels of β-hCG in the serum and cerebrospinal fluid [5]. The main treatment strategies for this tumor are radiotherapy and pre-radiation chemotherapy [5]. Patients with intracranial germinomas can present various symptoms, such as diabetes insipidus, growth retardation, precocious puberty, headache, visual disturbance, gait abnormalities, and motor weakness [5]. Involuntary movements, such as dystonia and choreoathetosis, have been reported in only a few patients with germinomas [5].
Pathological MMs without underlying structural abnormalities or specific syndromes (e.g., Klippel-Feil syndrome, Kallmann syndrome, agenesis of the corpus callosum, Arnold-Chiari malformation, or cleft spine) are associated with haploinsufficiency of some causative genes, namely, DCC, RAD51, and netrin-1 [1,2]. Late-onset MMs are associated with degenerative or acquired diseases or trauma, such as Parkinson’s disease, cerebrovascular diseases, craniovertebral damage, or (very rarely) brain tumors [1-4]. The brain lesions associated with late-onset MMs mostly involve the corpus callosum and primary motor cortex (M1) and occasionally secondary motor areas (e.g., the supplementary motor cortex, dorsal premotor cortex, and basal ganglia) [1-4].
Three hypotheses have been proposed for the development of MMs. Per the first hypothesis, MM develops owing to the abnormal development or decussation of the corticospinal tract (mainly in the medulla or at the upper cervical level). The second hypothesis states that MM develops owing to the imbalanced interhemispheric inhibition concomitant with bilateral activation of the M1. The third hypothesis is that MM develops owing to the abnormal bilateral delivery of signals to the M1 from the secondary motor areas [1,2]. None of these hypotheses have been confirmed; however, the first two hypotheses are commonly reported [1-4].
The loss of interhemispheric inhibition, which leads to the development of MMs, is identified in patients with defects of the corpus callosum (e.g., agenesis/dysgenesis, traumatic damage, or tumor invasion) or lesions in M1 such as infarction, trauma, or tumor [1-4]. Kaulen and Baehring [3] described a patient with MMs in the contralateral hand owing to a scar or residual tumor in the right corpus callosum and corona radiata at 1 year after right frontoparietal oligoastrocytoma surgery. In this patient, the development of MMs was attributed to the loss of interhemispheric inhibitory input to the ipsilateral M1 via the damaged areas of the ipsilateral corpus callosum [3].
Concomitant bilateral facilitation of the M1 owing to abnormally delivered signals from the secondary motor areas has been recognized in a few patients with MMs [1]. This mechanism may also apply in our case. In our case, the germinoma in the right basal ganglia may have activated the contralateral M1 in response to ipsilateral M1 activation. Therefore, our case was characterized by MMs in the ipsilateral hand concomitant with a right basal ganglia germinoma. Unfortunately, this hypothesis could not be proved in our case as functional brain MRI scans were not available for our patient.
To the best of our knowledge, this is the first case of a brain tumor presenting with only MMs, and is a rare report of MMs associated with the unilateral basal ganglia lesion.
Informed consent was waived by the Institutional Review Board of Chonnam National Unuversity Hospital (CNUH-EXP-2020-296) due to a retrospective nature of our study.
Notes
Young Ok Kim is an editorial board member of the journal, but she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Data curation: HC and YOK. Formal analysis: YOK. Methodology: SKK, KHL, HJB, and YOK. Visualization: SKK and KHL. Writing-original draft: HC and YOK. Writing-review & editing: YOK.
